��Ŀ����
����¡�ġ�ɱ�˺���(���˹��)����1986��ʹ1800������������һҹ֮��������ȥ����ѧ�ҿ��췢�֣������и���ɽ�ڣ������˴�������������ж����壬��������200 m��ĺ���ÿ������ˮ�о��ܽ���8 m3���ж����塣Ŀǰ�Ƽ���Ա���ҵ�һ����Ч�İ취�������ж����������Դ�����Ϊ�˲ⶨ�Ӻ���ȡ����ˮ����SO2�ĺ���������һ������ˮ���м�������ĵ⣬������֪Ũ�ȵ������������Һ�ζ������ĵ⣬�÷�ӦΪ��2Na2S2O3+I2====2NaI+Na2S4O6
����������⣺
(1)���ˮ����SO2��Ӧ�Ļ�ѧ����ʽΪ(��ʾ���÷�Ӧ��I2����������SO2����ԭ��) ____________________________________��
(2)����������Ƶζ������ĵ�ʱ����ѡ��______________��ָʾ��������Һ_____________(������)��ﵽ�ζ��յ㡣
(3)����20 mLˮ���м����W g����ȥV mL c mol��L-1��Na2S2O3��Һ����ˮ����SO2�����ʵ���Ũ���Ƕ��٣�
������(1)SO2���ˮ����������ԭ��Ӧ������H2SO4��HI��
(2)����ⵥ�ʣ�һ�㿼���õ�����Һ�����顣
(3)��SO2��Ӧʣ���n(I2)��
2Na2S2O3 �� I2
2 mol 1 mol
V��10-3 L��c mol��L-1 n(I2)
![]()
![]()
SO2 �� I2
1 mol 1 mol
n(SO2) (![]() ) mol
) mol
![]()
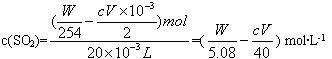
�𰸣�(1)I2+SO2+2H2O====H2SO4+2HI
(2)������Һ ����ɫ��Ϊ��ɫ
(3)(![]() ) mol��L-1
) mol��L-1

 ��У����ϵ�д�
��У����ϵ�д�
