��Ŀ����
��������������Ⱦ��Ϊ���أ����������Ϊ����ü�����⣮
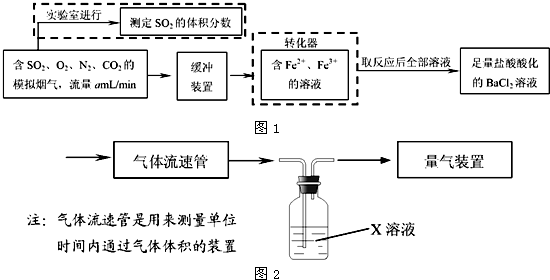
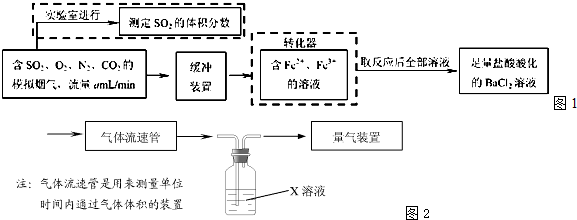
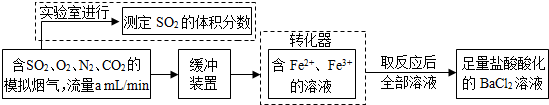
��1����ѧ���������Fe2+��Fe3+�����ӵĴ����ã������½�SO2������SO
��ʵ��SO2�Ļ������ã�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO
��ת���ʣ�
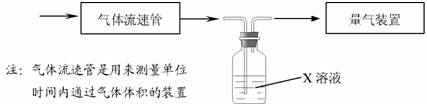
�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ�����ǣ�����ţ� ��

A����ĵ�����Һ B�����Ը��������Һ C������������Һ D���Ȼ�����Һ
��������ʵ�����ڱ�״���½��еģ�X����֪��������ʵ���Ũ�ȵ���Һ�����ⶨת������SO2������
��ת���ʣ���֪�������٣�����ⶨ�������� ��
��2�����Ͱ����������������ð�����������SO2����������狀���������泥�����һ������������з�Ӧ���ڷ�Ӧ����SO2��Ļ������ͨ�������İ����õ�һ�ֲ�Ʒ���ü������ŵ��� ��
��3��Ϊ��һ������SO2����Ⱦ�����Ϊ������������̽����CO��ԭSO2�õ�������ķ�������ȥSO2���÷����漰���Ļ�ѧ��ӦΪ��SO2+2CO=2CO2+
Sx��CO+
Sx=COS��2COS+SO2=2CO2+
Sx������COS�С�C�����ϼ�Ϊ ��
��4����������ˮ�к������ĺ��������ͨ��������Ĥ�ѵ����ս��д�����������ϸ���������½�NH
����ΪNO
��NH
+2O2=NO
+2H++H2O��Ȼ�����״���NO
�ͼ״�ת��Ϊ���������壮��д������״���Ӧ�����ӷ���ʽ ��
��1����ѧ���������Fe2+��Fe3+�����ӵĴ����ã������½�SO2������SO
2- 4 |
2- 4 |

�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ�����ǣ�����ţ�

A����ĵ�����Һ B�����Ը��������Һ C������������Һ D���Ȼ�����Һ
��������ʵ�����ڱ�״���½��еģ�X����֪��������ʵ���Ũ�ȵ���Һ�����ⶨת������SO2������
2- 4 |
��2�����Ͱ����������������ð�����������SO2����������狀���������泥�����һ������������з�Ӧ���ڷ�Ӧ����SO2��Ļ������ͨ�������İ����õ�һ�ֲ�Ʒ���ü������ŵ���
��3��Ϊ��һ������SO2����Ⱦ�����Ϊ������������̽����CO��ԭSO2�õ�������ķ�������ȥSO2���÷����漰���Ļ�ѧ��ӦΪ��SO2+2CO=2CO2+
| 1 |
| x |
| 1 |
| x |
| 3 |
| x |
��4����������ˮ�к������ĺ��������ͨ��������Ĥ�ѵ����ս��д�����������ϸ���������½�NH
+ 4 |
- 3 |
+ 4 |
- 3 |
- 3 |
��������1���ⶨģ��������SO2������������ɸ��ݶ�����������Ļ�ԭ�ԣ�����������Ե����Ը�����ػ��ˮ��Ӧ��ͨ����ɫ�ı仯�жϣ����ⶨת������SO2������SO42-��ת���ʣ�����Ҫ�ⶨʱ������ɳ�����������
��2�����ð�����������SO2����������狀���������泥�����Ϊ���ϣ�������Ⱦ��
��3��C�ķǽ�����С��O��S���ṹʽΪO=C=S��C�Ļ��ϼ�Ϊ+4�ۣ�
��4�����������Ϣ����������Ԫ�ط����ж�����������Ϊ������̼�͵��������ԭ���غ�д����ѧ����ʽ��
��2�����ð�����������SO2����������狀���������泥�����Ϊ���ϣ�������Ⱦ��
��3��C�ķǽ�����С��O��S���ṹʽΪO=C=S��C�Ļ��ϼ�Ϊ+4�ۣ�
��4�����������Ϣ����������Ԫ�ط����ж�����������Ϊ������̼�͵��������ԭ���غ�д����ѧ����ʽ��
����⣺��1���ٲⶨģ��������SO2������������ɸ��ݶ�����������Ļ�ԭ�ԣ�����������Ե����Ը�����ػ��ˮ��Ӧ��ͨ����ɫ�ı仯�жϣ�ѡ����AB���ϣ�������ɫ���Ҷ����������ԣ������������Ӧ��
�ʴ�Ϊ��AB��
�����ⶨת������SO2������SO42-��ת���ʣ�����Ҫ�ⶨʱ������ɳ������������ʴ�Ϊ��ʵ��ʱ��ͼ��������ữ���Ȼ�����Һ�����ɳ�����������
��2�����ð�����������SO2����������狀���������泥�����Ϊ���ϣ�������Ⱦ���ﵽ���������Դ��Ŀ�ģ�
�ʴ�Ϊ�����Ϊ�ʣ����������Դ��������Ⱦ��
��3��C�ķǽ�����С��O��S���ṹʽΪO=C=S��C�Ļ��ϼ�Ϊ+4�ۣ��ʴ�Ϊ��+4��
��4������ϸ���������½�NH4+����ΪNO3-��NH4++2O2=NO3-+2H++H2O��Ȼ���ڸ�������Һ��������Һ������ǿ�����ԣ�����״�����������Ӻ��������������״�Ϊ������Ϊ������̼��NO3-�ͼ״�ת��Ϊ���������壬�ƶ����ᱵ��ԭΪ��������Ӧ�����ӷ���ʽΪ6NO3-+5CH3OH+6H+=3N2��+5CO2��+13H2O��
�ʴ�Ϊ��6NO3-+5CH3OH+6H+=3N2��+5CO2��+13H2O��
�ʴ�Ϊ��AB��
�����ⶨת������SO2������SO42-��ת���ʣ�����Ҫ�ⶨʱ������ɳ������������ʴ�Ϊ��ʵ��ʱ��ͼ��������ữ���Ȼ�����Һ�����ɳ�����������
��2�����ð�����������SO2����������狀���������泥�����Ϊ���ϣ�������Ⱦ���ﵽ���������Դ��Ŀ�ģ�
�ʴ�Ϊ�����Ϊ�ʣ����������Դ��������Ⱦ��
��3��C�ķǽ�����С��O��S���ṹʽΪO=C=S��C�Ļ��ϼ�Ϊ+4�ۣ��ʴ�Ϊ��+4��
��4������ϸ���������½�NH4+����ΪNO3-��NH4++2O2=NO3-+2H++H2O��Ȼ���ڸ�������Һ��������Һ������ǿ�����ԣ�����״�����������Ӻ��������������״�Ϊ������Ϊ������̼��NO3-�ͼ״�ת��Ϊ���������壬�ƶ����ᱵ��ԭΪ��������Ӧ�����ӷ���ʽΪ6NO3-+5CH3OH+6H+=3N2��+5CO2��+13H2O��
�ʴ�Ϊ��6NO3-+5CH3OH+6H+=3N2��+5CO2��+13H2O��
���������⿼�����������ԭ��̽������Ŀ�Ѷ��еȣ�����ע�⣨1����3��Ϊ�״��㣬ע�������������ʣ��Լ�COS�Ľṹ��

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ