��Ŀ����
��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ�����Ca��OH��2����Һ�ʼ��ԣ�������һ�ص��ѧ�ҷ����˵綯�ƣ�E������ˮ�����ʱ�䣬�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��
2Cu+Ag2O=Cu2O+2Ag�������й�˵������ȷ���ǣ�������
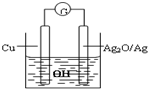
2Cu+Ag2O=Cu2O+2Ag�������й�˵������ȷ���ǣ�������
| A����ҵ���Ʊ���ͨˮ�����Ҫԭ���������ʯ��ʯ |
| B������ԭ��װ��ͼ�У�Ag2O/Ag������������Ӧ |
| C�������ĵ缫��ӦʽΪ��2Cu+2OH--2e-=Cu2O+H2O |
| D����ˮ��̻������У���������ˮ���ӵļ��٣���Һ�и�����Ũ�ȵı仯���µ綯�Ʊ仯 |

A��������ˮ����ڹ����ι�ҵ����ҵ���Ʊ���ͨˮ�����Ҫԭ���Ǻ���ʯ��ʯ�����ε�ճ������A��ȷ��
B�����ݵ�ط�Ӧʽ2Cu+Ag2O=Cu2O+2Ag֪��Ag2O/Ag���������������ϵõ��ӷ�����ԭ��Ӧ����B����
C�����ݵ�ط�Ӧʽ2Cu+Ag2O=Cu2O+2Ag֪��ͭʧ�����������������Ϸ����ĵ缫��ӦʽΪ��2Cu+2OH--2e-=Cu2O+H2O����B��ȷ��
D��ˮ��̻������У�����ˮ���Ӽ��٣��ܼ��������ٵ�����Һ�и�����Ũ�ȵı仯���Ӷ�����綯�Ʊ仯����D��ȷ��
��ѡB��
B�����ݵ�ط�Ӧʽ2Cu+Ag2O=Cu2O+2Ag֪��Ag2O/Ag���������������ϵõ��ӷ�����ԭ��Ӧ����B����
C�����ݵ�ط�Ӧʽ2Cu+Ag2O=Cu2O+2Ag֪��ͭʧ�����������������Ϸ����ĵ缫��ӦʽΪ��2Cu+2OH--2e-=Cu2O+H2O����B��ȷ��
D��ˮ��̻������У�����ˮ���Ӽ��٣��ܼ��������ٵ�����Һ�и�����Ũ�ȵı仯���Ӷ�����綯�Ʊ仯����D��ȷ��
��ѡB��

��ϰ��ϵ�д�
�����Ŀ
 ��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ��������壬��Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O?Cu2O+2Ag�������й�˵����ȷ���ǣ�������
��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ��������壬��Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O?Cu2O+2Ag�������й�˵����ȷ���ǣ�������| A��ˮ����һ�ֹ����ι�ҵ��Ʒ | B������ԭ��ʾ��ͼ�У�Ag2OΪ���� | C�������ĵ缫��ӦʽΪ��2Cu+2OH--2e-?Cu2O+H2O | D����ع���ʱ��OH-�������ƶ� |
 ��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ�����Ca��OH��2����Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O�TCu2O+2Ag�������й�˵����ȷ���ǣ�������
��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ�����Ca��OH��2����Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O�TCu2O+2Ag�������й�˵����ȷ���ǣ������� ��ͨˮ���ڹ̻�����������ˮ���Ӽ��٣�������Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O=Cu2O+2Ag�������й�˵����ȷ���ǣ�������
��ͨˮ���ڹ̻�����������ˮ���Ӽ��٣�������Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O=Cu2O+2Ag�������й�˵����ȷ���ǣ������� ��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ�����Ca��OH��2����Һ�ʼ��ԣ�������һ�ص��ѧ�ҷ����˵綯�ƣ�E������ˮ�����ʱ�䣬�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��
��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ�����Ca��OH��2����Һ�ʼ��ԣ�������һ�ص��ѧ�ҷ����˵綯�ƣ�E������ˮ�����ʱ�䣬�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ��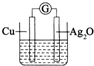 ��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ�����OH-����Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ������ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O�TCu2O+2Ag�������й�˵������ȷ���ǣ�������
��ͨˮ���ڹ̻�����������ˮ���Ӽ��ٲ�����OH-����Һ�ʼ��ԣ�������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ�����ʱ�䣮�˷���ԭ������ͼ��ʾ����Ӧ���ܷ���ʽΪ��2Cu+Ag2O�TCu2O+2Ag�������й�˵������ȷ���ǣ�������