��Ŀ����
ij��ȤС��Ϊ��֤�ճ������õĻ��ͷ�Ϻ���KClO3��MnO2��S�����������ʵ������ͼ��

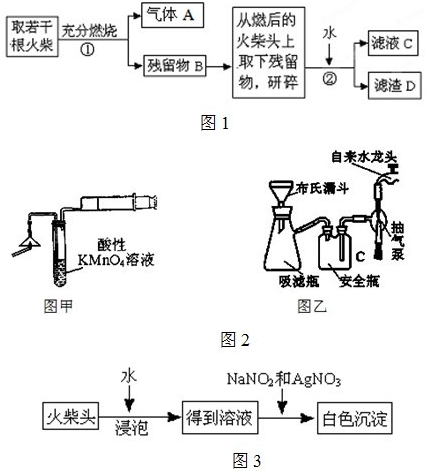
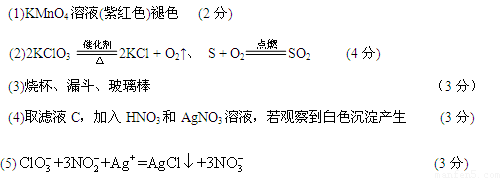
��1��Ϊ����֤����A������ͼ��ʾ����ʵ�飺���ܹ۲쵽 ������֤�����ͷ�Ϻ���SԪ�ء�
��2��������з����������ص�������Ҫ��Ӧ�ķ���ʽ�� ��
��
��3����������������������� ��
��4��Ҫ֤�����ͷ�к�����Ԫ�صĺ���ʵ�鲽����
��
��5����ѧ������˼�����ͷ������ص���һ���е�ʵ�鷽����
���йط�Ӧ�����ӷ���ʽΪ ��
����������

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д���������dz��õ�������Ʒ��ij��ȤС��Ϊ��֤�ճ������õĻ��ͷ�Ϻ���KClO3��MnO2��S�����ʣ����������ϵ��ʵ�飬���ش����и��⡣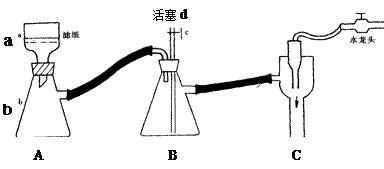
���ͷ����Ԫ�ص�ʵ��֤��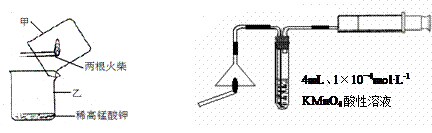
A B
�����װ��A�пɹ۲쵽 ��ʵ��������֤�����ͷ�Ϻ���SԪ�ء�
�Ƽ�ͬѧ��ΪBװ���г��˿�ѡ��ϡ����������ѡ�ã�Ʒ����Һ����ɫʯ����Һ�����з�̪��NaOH��Һ����ˮ�ȣ�����ҷ�������̭����ɫʯ����Һ�͵��з�̪��NaOH ��Һ������Ϊ��̭��ԭ������� ����ͬѧ����Ϊ��Aװ����ѡ��Ũ�ĸ������������Һ���ã���ΪŨ��Խ��Ӧ����Խ�죬����Ϊ���� �����ж���˵��ԭ��
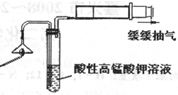
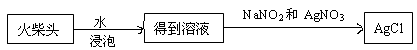
���ͷ�к���ClԪ�ص�֤�������������ʵ������ͼ��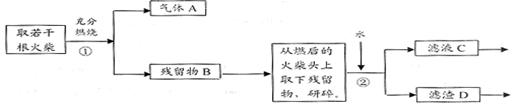
��Ϊ��֤���ͷ�к���ClԪ�ص�ʵ�鲽���ǣ�ȡ��ҺC������ش����ʵ�鲽�� �� ��
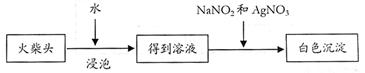
����ѧ�����������ͷ��KClO3��һ��ʵ�鷽����
|
|

�Ų���ڵ�ʵ�����װ������ͼ��ʾ������a������Ϊ ��װ��B�������� ��
������ϻ���;ֹͣ����ʱ����ѵ���ȷ������ ��
�Ƶõ��ij�������95%�ľƾ�ϴ���ٳ��ˣ������þƾ�ϴ�ӵ�ԭ�� ����3��ʵ��� �û��ͷ��KClO3����������Ϊ ��δϴ�ӳ��������KClO3������������ ���� ��ƫ����ƫС��������Ӱ�족����ͬ����