��Ŀ����
��12�֣�ij�л�������A�����ϣ��������к�̼Ϊ70.59��������Ϊ 5.88�������ຬ�������������з����ⶨ���л����������Է��������ͷ��ӽṹ��
����һ��������������֪A����Է�������Ϊ136��
���������˴Ź����Dz��A�ĺ˴Ź���������4���壬�����֮��Ϊ1��2��2��3������ͼA��
�����������ú�������Dz��A���ӵĺ����������ͼB��

(1)�����й��� �ֻ�ѧ������ͬ����ԭ�ӣ�
(2) A�ķ���ʽΪ ��
(3) ������������һ���л���_____________ ��
(4)A�ķ�����ֻ��һ������������___________________�������)��
a. A����Է������� b. A�ķ���ʽ
c. A�ĺ˴Ź�������ͼ d. A���ӵĺ������ͼ
(5)A�Ľṹ��ʽΪ ��
(6)A�ķ�����ͬ���칹���ж��֣�������ͬʱ�����������������ܷ���ˮ�ⷴӦ���ڷ��ӽṹ�к���һ�����������A��ͬ���칹�干��_______�֡�
��12�֣�(1) 4 (2) C8H8O2 ��(3) ���� ��(4) ___bc__ ��
(5) C6H5COOCH3 (6) ______4______
��������
�����������1���ɺ˴Ź�������֪A��4���壬����A�����й���4�ֻ�ѧ������ͬ����ԭ�ӣ���2����֪A�к�̼Ϊ70.59��������Ϊ 5.88��������23.53������ʽ����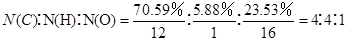 �����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ��C4H4O��n ������A����Է�������Ϊ136���ɵ�n=2������ʽΪC8H8O2��
�����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ��C4H4O��n ������A����Է�������Ϊ136���ɵ�n=2������ʽΪC8H8O2��
��3���Ӻ������֪8��̼ԭ������6��C�ڱ����У�����C=O��C��O��C��C��H��Щ���ţ����Ʋ⺬�еĹ�����Ϊ����������A�����ࣻ��4����A�ĺ˴Ź���������4���壬�����֮��Ϊ1��2��2��3��A�ķ���ʽΪC8H8O2����֪A������ֻ��һ��������Ϊ���ϵ���ԭ����3����(5)�����������Ƴ�A�Ľṹ��ʽΪC6H5COOCH3��
(6)A�ķ�����ͬ���칹���ж��֣��ܷ���ˮ�ⷴӦ��һ���������������ţ��ַ��ӽṹ�к���һ���������Ը���A��ͬ���칹����CH3����C6H4OOCH�������б�������λ����λ����λ���ֽṹ���ټ���C6H5COOCH3 �����Թ���4�ֽṹ��
���㣺���⿼��ⶨ���л����������Է��������ͷ��ӽṹ��������������еõ����ӵ���Է���������������˴Ź���ͼ��֪�л����������Ļ�������������������ȷ���������������ţ��ۺϼ�����Եõ��л���ķ���ʽ�ͽṹ��
��������Ϥ�����Ƚ����������ã��Ӷ����ղⶨ�л�����Ӻͽṹ�ķ���

 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�


