��Ŀ����
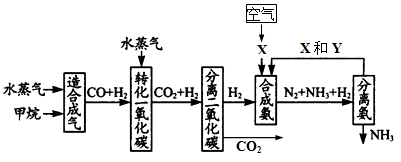
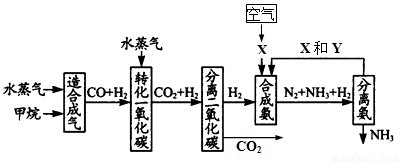
(17��) �����зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳɰ�����Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����
����д���пհף�
(1)��֪2mol������ˮ������t�桢p kPaʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
(2)ͼ��XΪ_____��YΪ_____���ѧʽ��������K2CO3��Һ���շ������CO2�������ӷ���ʽΪ_____________________________________
(3)�ںϳɰ���ҵ�У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ��������������û�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�_______________________________��
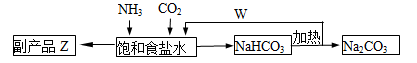
(4)�����Ƽ�У��ϳɰ�������NH3��CO2ͨ�뱥��ʳ��ˮ���տ��Ƶô����ͼ��ʾ
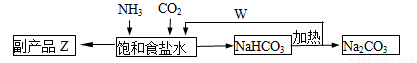
��Ӧ����ʳ��ˮͨ����_______���壨д��ѧʽ����ͬ��������ƷZΪ______��������______��WΪ_______��
�������� Na2CO3 5.3�֣����������ٿ��Ƶø���ƷZ_______�֡�
��17�֣�
��1��CH4 (g) + H2O (g) === CO(g) + 3H2(g) ��H = + 1/2a kJ/mol (3��)
��2��N2 �� H2 ��ÿ��1�֣���2��)
CO3�� + CO2 + H2O = 2HCO3�� (3��)
��3����С������Ũ�ȣ��ٽ�ƽ�������ƶ� (2��)
��4����NH3��NH4Cl ���ʣ� CO2 (ÿ��1�֣���4��)
�� 5.35�� (3��)
��������