��Ŀ����
18����298K1.0l��l05Pa�£���32gSO2ͨ��750mL 1mol/L KOH��Һ�г�ַ�Ӧ����÷�Ӧ�ų�xkJ����������֪�ڸ������£�lmolSO2ͨ��1L 2mol/L KOH��Һ�г�ַ�Ӧ�ų�ykJ����������SO2��KOH��Һ��Ӧ����KHSO3���Ȼ�ѧ����ʽ��ȷ���ǣ�������| A�� | SO2��g��+KOH��aq���TKHSO3��aq����H=-��4x-y��kJ/mol | |
| B�� | SO2��g��+KOH��aq���TKHSO3��aq����H=-��2x-y��kJ/mol | |
| C�� | SO2��g��+KOH��aq���TKHSO3��aq����H=-��2y-x��kJ/mol | |
| D�� | 2SO2��g��+2KOH��1���T2KHSO3��1����H=-��8x-2y��kJ/mol |
���� ��298K��1.01��105Pa�£���32g SO2ͨ��750mL 1mol/L KOH��Һ�г�ַ�Ӧ����÷�Ӧ�ų�x kJ����������2molSO2��3molKOH������Ӧ���Ȼ�ѧ����ʽΪ2SO2+3KOH=KHSO3+K2SO3+H2O����H=4xkJ/mol��1mol SO2ͨ��1L 2mol/L KOH��Һ�г�ַ�Ӧ�ų�y kJ����������1molSO2��2molKOH������Ӧ���Ȼ�ѧ����ʽΪSO2+2KOH=K2SO3+H2O����H=ykJ/mol�����ݸ�˹���ɴ��⣮
��� �⣺��298K��1.01��105Pa�£���32g SO2ͨ��750mL 1mol/L KOH��Һ�г�ַ�Ӧ����÷�Ӧ�ų�x kJ����������2molSO2��3molKOH������Ӧ���Ȼ�ѧ����ʽΪ��2SO2��g��+3KOH��aq��=KHSO3��aq��+K2SO3��aq��+H2O��l������H=-4xkJ/mol��1mol SO2ͨ��1L 2mol/L KOH��Һ�г�ַ�Ӧ�ų�y kJ����������1molSO2��2molKOH������Ӧ���Ȼ�ѧ����ʽΪ��SO2��g��+2KOH��aq��=K2SO3��aq��+H2O��l������H=-ykJ/mol�����ݸ�˹���ɽ���-�ڵ�SO2��KOH��Һ��Ӧ����KHSO3���Ȼ�ѧ����ʽΪSO2��g��+KOH��aq��=KHSO3��aq������H=-��4x-y��kJ/mol����SO2��g��+KOH��aq��=KHSO3��aq������H=-��4x-y��kJ/mol��
��ѡA��
���� ���⿼���Ȼ�ѧ����ʽ����д�ͼ��㣬�Ǹ߿��г������ͣ��������漰����ѧ����ʽ���йؼ��������˹���ɵ��й�Ӧ�ã���һ�ص����ͣ�ѧ����Ӧע������д�Ȼ�ѧ����ʽʱ���������ʵ�״̬��

| A�� | ú��ʯ�еĽ��������������ˮ���Ҷ�����ˮ��Ӧ | |
| B�� | ú��ʯ���е������������Ӧ��ˮ�����У�������ǿ����H3PO4 | |
| C�� | ú��ʯ������������ʯˮ�ࡢ�ͻ�ש�Ƚ������� | |
| D�� | ����������ܽ�ú��ʯ�е����������� |
| A�� | ���ʵ�����һ�������������ʵ����ĵ�λ��Ħ�� | |
| B�� | 2 molˮ��Ħ��������1 molˮ��Ħ��������2�� | |
| C�� | N2��CO��ɵĻ������28g�������ʵ���Ϊ1mol | |
| D�� | ��״���£�2L������̼��3Lһ����̼�����е�ԭ����Ŀ��ȣ� |
| A�� | 0.1mol/L | B�� | 0.5mol/L | C�� | 4mol/L | D�� | 1mol/L |
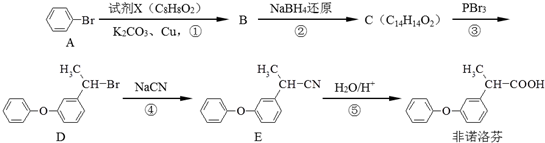
 ��C��
��C��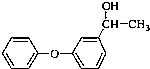 ��
��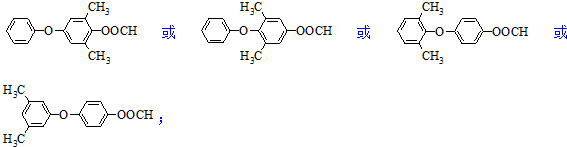 ��
��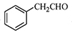 Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ���£�
�ĺϳ�·������ͼ���£�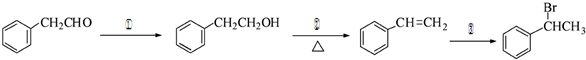
 ̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�ã�����ϳ������裬����ͭ�������Ȼ����
̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�ã�����ϳ������裬����ͭ�������Ȼ����