��Ŀ����
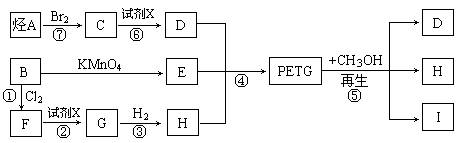
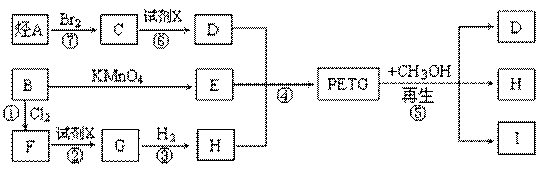
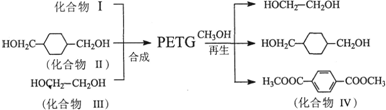
������(PETG)��������Ĺ���ȡ����ʺͿɻ��������õ��ص㣬�㷺Ӧ����ҽ����Ʒ���ճ�����Ʒ�ͻ�ױƷ��װ����ҵ��PETG�Ľṹ��ʽΪ��

PETG�²��ϵĺϳɺ�����·�����£�

�Իش��������⣺
��1��������IV�ķ���ʽΪ_______________________________��
��2��������I�Ľṹ��ʽ��______________��������II��һ��ͬ���칹��V���뱥��NaHCO3��Һ��Ӧ�ų�CO2�ҷ��ӽṹ�к���5����������V�Ľṹ��ʽΪ__________��
��3���ϳɵķ�Ӧ����Ϊ____________________________��
��4��������������ϩ��Br2ͨ�������ӳɺõ��IJ�����һ�������·���ȡ����Ӧ����ã���д������ȡ����Ӧ�Ļ�ѧ����ʽ��______________________________��
��5����һ�������£�CH3OH����̼����ϩ�� ��������PETG�����ķ�Ӧ�����в���֮һΪ̼�������[��ѧʽΪ(CH3O)2CO��һ�������Ļ���ԭ��]��д����Ӧ��ѧ����ʽ�����ñ귴Ӧ��������____________________��
��������PETG�����ķ�Ӧ�����в���֮һΪ̼�������[��ѧʽΪ(CH3O)2CO��һ�������Ļ���ԭ��]��д����Ӧ��ѧ����ʽ�����ñ귴Ӧ��������____________________��
��16�֣���1��C10H10O4��2�֣�
��2�� ��ÿ��3�֣���6�֣�
��ÿ��3�֣���6�֣�
��3�����۷�Ӧ��2�֣�
��4��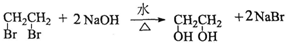 ��3�֣�
��3�֣�
��5��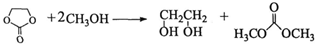 ��3�֣�
��3�֣�
��������
�����������1���۲컯����IV�Ľṹ��ʽ����������H3COOC�\���\C6H4�\���\COOCH3�����ֹ��ɣ���IV�ķ���ʽΪC10H10O4����2���۲�PETG�Ľṹ��ʽ���������Ƕ�Ԫ������Ԫ���ᷢ�����۷�Ӧ�õ��ĸ߷��ӻ����ȥ�������š��ۺ϶ȣ�m��n��֮�Ͽ�����������̼���������ֱ����ԭ�Ӳ���ԭ�ӡ�̼ԭ�Ӳ��ǻ����͵õ��������ֵ��壬�ɴ˿��ƶ��仯����I�ǶԱ�������۲컯����II�Ľṹ��ʽ����������HOH2C�\���\C6H10�\���\CH2OH���ɣ���II�ķ���ʽΪC8H16O2���뱥��̼������Һ��Ӧ�ų�������̼���壬˵����ͬ���칹�庬���Ȼ����\COOH�������Ȼ�����Ǻ���5������ԭ���ţ��\C7H15������\C7H15�Ľṹ��ʽΪCH3C(CH3)2C(CH3)2�\�����Ի�����V�Ľṹ��ʽΪCH3C(CH3)2C(CH3)2COOH����3��mĦIII��mĦI��nĦII��nĦI�������۷�Ӧ������1ĦPETG�ͣ�2m+2n�\1��Ħˮ����4����ϩ��CH2=CH2����Br2�����ӳɷ�Ӧ���õ�1��2���������飻±������NaOHˮ��Һ����ʱ����ȡ����Ӧ��ˮ�ⷴӦ�����ɴ���±���ơ�ˮ����CH2BrCH2Br+2NaOH��HOCH2CH2OH+2NaBr����5���۲�PETG��CH3OH�����ķ�Ӧ������PETG��ˮ��Ϊ���ֵ��壬���еĶԱ�����������״�����������Ӧ���õ�������IV��ˮ��̼����ϩ���Ľṹ��ʽ�к���2����������1��̼����ϩ������2��H2O����ˮ�ⷴӦ���õ�1��HOCH2CH2OH��1��H2CO3��Ȼ��1��H2CO3��2��CH3OH����������Ӧ���õ�1��̼���������2��H2O���ɴ˿��ƶϻ��д���÷�Ӧ�Ļ�ѧ����ʽ��
���㣺�����л��ϳ����ƶϴ��⣬�漰�л���ķ���ʽ���ṹ��ʽ����Ӧ���͡���ѧ����ʽ���ƶϵ�֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�������������Դ�������ȷ������Ź㷺����;��ʪ�����ɷ��Ʊ��������ε�ԭ�����±���ʾ��
|
ʪ�� |
ǿ���Խ����У�Fe(NO3)3��NaClO��Ӧ�����Ϻ�ɫ����������Һ |
|
�ɷ� |
Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫ�������κ�KNO2�Ȳ��� |
��1����ҵ����ʪ���Ʊ�������أ�K2FeO4������������ͼ��ʾ��
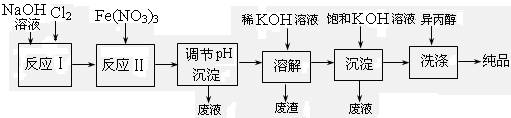
|
�ٷ�ӦI�Ļ�ѧ����ʽΪ���� ��
�ڷ�ӦII�����ӷ���ʽΪ���� ��
�ۼ��뱥��KOH��Һ��Ŀ�������� ��
��2�����������һ�������ˮ���������䴦��ˮ
 ��ԭ��Ϊ ��__________��
��ԭ��Ϊ ��__________��
��3���ɷ��Ʊ�K2FeO4�ķ�Ӧ�У���������
��ԭ�������ʵ���֮��Ϊ���� ��
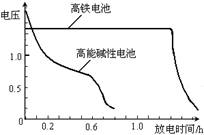
��4��������������������еĿɳ��ɵ�أ�
��ͼΪ�õ�غͳ��õĸ��ܼ��Ե�ص�
�ŵ����ߣ��ɴ˿ɵó��ĸ�����ص���
�������� ������ ��

 ��������PETG�����ķ�Ӧ�����в���֮һΪ̼�������[��ѧʽΪ��CH3O��2CO��һ�������Ļ���ԭ��]��д����Ӧ��ѧ����ʽ�����ñ귴Ӧ������
��������PETG�����ķ�Ӧ�����в���֮һΪ̼�������[��ѧʽΪ��CH3O��2CO��һ�������Ļ���ԭ��]��д����Ӧ��ѧ����ʽ�����ñ귴Ӧ������