��Ŀ����
ij��ɫ�����������п��ܴ��ڵ��������£�Na+��Ag+��Fe3+��Ba2+��Al3+�� 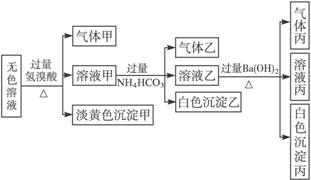
(1)��������____________(�ѧʽ)�����ɳ��������ӷ���ʽΪ____________________��
(2)��������____________(�ѧʽ)������Һ�����ɳ����ҵ����ӷ���ʽΪ____________��
��3��������������____________(�ѧʽ)��
(4)����ijɷ����ļ��ֿ��ܣ��𣺢�____________����____________����____________��(�ж����־�������֣��ɲ�����)
(5)�ۺ�������Ϣ�����Կ϶����ڵ�������____________��
(1)S 2S2-+![]() +6H+=====3S��+3H2O
+6H+=====3S��+3H2O
(2)Al(OH)3 Al3++3![]() ====Al(OH)3��+3CO2��
====Al(OH)3��+3CO2��
(3)BaCO3��BaCO3��BaSO4
(4)��H2S ��SO2 (5)S2-��![]() ��
��![]() ��Na+
��Na+
��������ɫ��Һ�в�����Fe3+��Ag+����Һ�е�������![]() ��S2-��
��S2-��![]() ��
��![]() �Ⱦ����ܴ������棬�ʵ���ɫ���������廯����ӦΪ����������Һ�к�S2-��
�Ⱦ����ܴ������棬�ʵ���ɫ���������廯����ӦΪ����������Һ�к�S2-��![]() ����Al3+��Ba2+���϶���Na+(��Һ�ʵ����ԣ�����Ӧ����һ��������)����NH4HCO3���ɰ�ɫ��������ԭ��Һ�к�
����Al3+��Ba2+���϶���Na+(��Һ�ʵ����ԣ�����Ӧ����һ��������)����NH4HCO3���ɰ�ɫ��������ԭ��Һ�к�![]() �������⣬�������п϶���BaCO3����ԭ��Һ����
�������⣬�������п϶���BaCO3����ԭ��Һ����![]() ����������л�������BaSO4���־ݵ�һ���ķ�Ӧʽ����Ӧ�п���S2-��
����������л�������BaSO4���־ݵ�һ���ķ�Ӧʽ����Ӧ�п���S2-��![]() �������ֱ��������ᷴӦ����H2S��SO2���ɡ�
�������ֱ��������ᷴӦ����H2S��SO2���ɡ�





