��Ŀ����
��15�֣��������[aFe2(SO4) 3��b(NH4) 2SO4��cH2O]�㷺���ڳ�����������ˮ����ҵѭ��ˮ�ľ��������ȡ�ij����������������������������ƣ��������Ϊԭ�ϣ���������¹���������ȡ������李�
��ش��������⣺
��1������������Һ��H2SO4�ữ����ҪĿ����____________________________������A����Ҫ�ɷ���__________________��
��2���������������ʺϵ�������B�� ����Ӧ�����ӷ���ʽ ��
a��NaClO b��H2O2 c��KMnO4 d��K2Cr2O7
��3�������ס��ҵ����Ʒֱ��ǣ���______________����___________________��
��4�����������У����������ʺϵ�������B����֮��ͼ�������֮ǰ����ȡ��������Fe2+�Ƿ���ȫ���������������Լ�Ϊ ��д���ƣ����ܷ������Ե�KMnO4��Һ�� ������ܣ����ʺ��ԣ��������ǣ� �����������ֻ�ʽ˵����
��5���������������NH4+�ķ����� ��
��6����ȡ14.00 g������Ʒ����������ˮ���Ƴ�100 mL��Һ���ֳ����ȷݣ�������һ���м�������NaOH��Һ������ϴ�ӵõ�2.14 g����������һ����Һ�м���0.05 mol Ba (NO3)2��Һ��ǡ����ȫ��Ӧ�����������淋Ļ�ѧʽΪ______________________��
��1��������Һ��SO42-Ũ�ȣ���Ca2+ת��Ϊ������ͬʱ����Fe2+�� Fe3+ˮ�⣻CaSO4.
��2��b;H2O2��2Fe2+��2H+��2Fe2+��2H2O
��3����ȴ�ᾧ����������
��4�����軯����Һ�����ܣ���Ϊ��������Ͷ��������Ӿ���ʹ���Ը��������Һ��ɫ
��5�����Թ��м���������Ʒ���������ƹ�����ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ����6��Fe2(SO4)3��2(NH4)2SO4��2H2O
���������������1��������Һ��SO42-Ũ�ȣ���Ca2+ת��Ϊ������ͬʱ����Fe2+�� Fe3+ˮ�⣻CaSO4.
��2��b����ɫ��������������Լ����������µ����ʣ�H2O2��2Fe2+��2H+��2Fe2+��2H2O
��3����ȴ�ᾧ����������
��4���������������Ӧ��ʹ�û�ɫ�����軯��K3��Fe(CN)6����Һ����������������������ӷ�Ӧ���ɴ���������ɫ�����軯����������3Fe2+��2��Fe(CN)6��3-��Fe3��Fe(CN)6��2�������ܣ���Ϊ��������Ͷ��������Ӿ���ʹ���Ը��������Һ��ɫ
��5�����Թ��м���������Ʒ���������ƹ�����ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ����֤��ԭ��Һ�к��а������ӡ�
��6��14.00 g������Ʒ�У������������Ƶõ�2.14 g����Ϊ��������������
n(Fe)��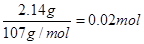
 ����һ����Һ�м���0.05 mol Ba (NO3)2��Һ��ǡ����ȫ��Ӧ�������ᱵ�����������Һ�е����������Ϊ0.05 mol ��Ҳ�������к��е�Fe2(SO4)3Ϊ0.01 mol��(NH4)2SO4Ϊ0.02 mol ����ʱ����Ϊ��0.01 mol ��400g/mol��0.02 mol ��132g/mol��6.64g��ʣ�µľ���H2O����������ôÿһ����Һ��ˮ������Ϊ��7.00g��6.64g��0.36g,n(H2O)��0.02 mol����˸�������淋Ļ�ѧʽΪ��Fe2(SO4)3��2(NH4)2SO4��2H2O
����һ����Һ�м���0.05 mol Ba (NO3)2��Һ��ǡ����ȫ��Ӧ�������ᱵ�����������Һ�е����������Ϊ0.05 mol ��Ҳ�������к��е�Fe2(SO4)3Ϊ0.01 mol��(NH4)2SO4Ϊ0.02 mol ����ʱ����Ϊ��0.01 mol ��400g/mol��0.02 mol ��132g/mol��6.64g��ʣ�µľ���H2O����������ôÿһ����Һ��ˮ������Ϊ��7.00g��6.64g��0.36g,n(H2O)��0.02 mol����˸�������淋Ļ�ѧʽΪ��Fe2(SO4)3��2(NH4)2SO4��2H2O
���㣺 �������ʵ��Ʊ��Լ��������ӵ����ʡ�

��ͼ����ʵ�����Ƶõ���ϩ(C2H5OH  CH2=CH2��+H2O)����ˮ������ȡ1,2-��������IJ���װ��ͼ������ͼʾ�ж�����˵����ȷ����( )
CH2=CH2��+H2O)����ˮ������ȡ1,2-��������IJ���װ��ͼ������ͼʾ�ж�����˵����ȷ����( )
| A��װ�âٺ�װ�â��ж�ʢ��ˮ����������ͬ |
| B��װ�âں�װ�â��ж�ʢ��NaOH��Һ�������յ�������ͬ |
| C��������÷�Һ�ķ������з��룬1,2-��������Ӧ�ӷ�Һ©�����Ͽڵ��� |
| D���Ʊ���ϩ������1,2-��������ķ�Ӧ���ͷֱ�����ȥ��Ӧ�ͼӳɷ�Ӧ |
����˵����ȷ����
| ѡ�� | ʵ�� | ���ͻ���� |
| A | �ýྻ��Pt˿պȡij��Һ������ɫ��Ӧ������ʻ�ɫ | ����Һ��һ������Na���� ��K�� |
| B | �ýྻ�IJ����������Na2O2����֬��������֬��ȼ�� | CO2��H2O��Na2O2��Ӧ�Ƿ��ȷ�Ӧ |
| C | ����ˮ�е���ֲ���ͣ����Ͳ�����ɫ | �岻������֬ |
| D | �������ữ��H2O2����Fe(NO3)2��Һ����Һ���ɫ | H2O2�������Ա�Fe3+ǿ |
�����������ʵ��ó��Ľ�����ȷ����
| ��� | ʵ�� | ���� |
| A | ��ij��Һ�м���ϡ���ᣬ������������ͨ�����ʯ��ˮ��ʯ��ˮ����� | ����Һһ����̼������Һ |
| B | �ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ | ����Һ��һ����������Һ |
| C | ��������Һ�м�ϡ���ᣬ����Ƭ�̣��μ�������Һ��ˮԡ���ȣ����������� | �õ���δ����ˮ�� |
| D | ��ij��Һ�еμ�KSCN��Һ����Һ����ɫ���μ���ˮ����Һ�Ժ�ɫ | ����Һ��һ����Fe2+ |
���г��ӷ����������
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A. | CO(g) | CO2(g) | NaOH ��Һ��ŨH2SO4 | ϴ�� |
| B. | NH4Cl(aq) | Fe3��(aq) | NaOH��Һ | ���� |
| C. | Cl2(g) | HCl(g) | ����ʳ��ˮ��ŨH2SO4 | ϴ�� |
| D. | Na2CO3(s) | NaHCO3(s) | �� | ���� |
����˵����ȷ����
| A��������������ͭ����Һ�ɼ���ʧȥ��ǩ���Ҵ�������������ȩ��������ƿ��ɫ��Һ |
| B�������������е���Ԫ��ʱ�����Ƚ���������������ữ���ټ���������Һ�����飬ͨ���۲��Ƿ��а�ɫ�������ж��Ƿ������Ԫ�� |
| C��Ħ�����Ʊ���������мҪ����̼������Һ����ϴ�ӣ�ʹ���ȵ�̼������Һ�������ȥ���۵���������������Һ����ʱ�������������� |
| D�����ڱ�������ˮ������Ӧʱ������Ӧ�࣬����ֻ��������ˮ�Ժ����ӵķ�ˮ�����Լ��飬�������������ⶨ |
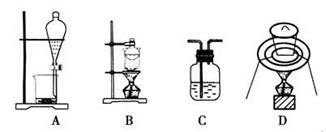
���Т١����ĸ�ͼ��ijѧϰС����Ƶ�������ʵ��Ʊ�װ�ã�������ȷ����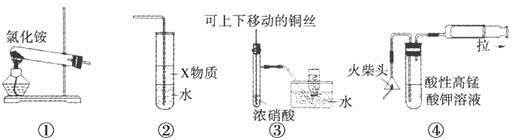
| A����װ�âٿ����Ʊ����� |
| B����װ�â���X����Ϊ����������ʵ�����Ʊ�������ˮ������ֹ�������� |
| C��װ�âۿ������Ʊ����ռ�����NO2���� |
| D��װ�âܿ��û��ͷȼ���Ʊ�����SO2�������������SO2 |
�ᴿ�����������ᱵ���ʵ��������Һ������ʹ�õķ���Ϊ (����)��
| A���������̼������Һ�����ˣ���ȥ��������Һ�в����������� |
| B����������������Һ�����ˣ���ȥ��������Һ�в����������� |
| C�����������������Һ�����ˣ���ȥ��������Һ�в����������� |
| D���������̼�����Һ�����ˣ���ȥ��������Һ�в����������� |