��Ŀ����
�ں����ܱ������н��з�Ӧ��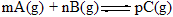 ����Ӧ��5���Ӵﵽƽ�⣬��ô�ʱA��Ũ�ȼ�С��a mol/L����C��Ũ��������2/3 a mol/L����֪ƽ����Ӧ���ʣ�v (C) =2v (B)����1��д��������ѧ����ʽ�и����ʵ�ϵ����m = ______��n = _______��p = _______��
����Ӧ��5���Ӵﵽƽ�⣬��ô�ʱA��Ũ�ȼ�С��a mol/L����C��Ũ��������2/3 a mol/L����֪ƽ����Ӧ���ʣ�v (C) =2v (B)����1��д��������ѧ����ʽ�и����ʵ�ϵ����m = ______��n = _______��p = _______��
��2��ѹǿһ��ʱ��C�İٷֺ������¶ȡ�ʱ�䣨T��ʾ�¶ȣ�t��ʾʱ�䣩�Ĺ�ϵ��ͼ��ʾ��
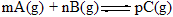 ����Ӧ��5���Ӵﵽƽ�⣬��ô�ʱA��Ũ�ȼ�С��a mol/L����C��Ũ��������2/3 a mol/L����֪ƽ����Ӧ���ʣ�v (C) =2v (B)����1��д��������ѧ����ʽ�и����ʵ�ϵ����m = ______��n = _______��p = _______��
����Ӧ��5���Ӵﵽƽ�⣬��ô�ʱA��Ũ�ȼ�С��a mol/L����C��Ũ��������2/3 a mol/L����֪ƽ����Ӧ���ʣ�v (C) =2v (B)����1��д��������ѧ����ʽ�и����ʵ�ϵ����m = ______��n = _______��p = _______����2��ѹǿһ��ʱ��C�İٷֺ������¶ȡ�ʱ�䣨T��ʾ�¶ȣ�t��ʾʱ�䣩�Ĺ�ϵ��ͼ��ʾ��
�ɴ˿�֪���÷�ӦΪ________������ȡ����ȡ�����Ӧ��
��3���÷�Ӧ��ƽ�ⳣ����ʾʽΪ____�������¶ȣ�Kֵ��___�����������С�����䡱����
��4������ͼ1��ͼ2����ʾ�÷�Ӧ��t1ʱ�ﵽƽ�⣬��t2ʱ�ı�ij���������仯�����ߡ�
��3���÷�Ӧ��ƽ�ⳣ����ʾʽΪ____�������¶ȣ�Kֵ��___�����������С�����䡱����
��4������ͼ1��ͼ2����ʾ�÷�Ӧ��t1ʱ�ﵽƽ�⣬��t2ʱ�ı�ij���������仯�����ߡ�
���жϣ�ͼ1��t2ʱ�ı��������______________��ͼ2��t2ʱ�ı��������____________��
��1��3��1��2
��2������
��3��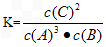 ����С
����С
��4��ʹ�ô����������¶�
��2������
��3��
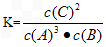 ����С
����С��4��ʹ�ô����������¶�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��ӦCO(g)+2H2(g)  2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
| ����� | 1 | 2 | 3 | 4 | |
| ��Ӧ�¶�/�� | 225 | 235 | 225 | 235 | |
| ��Ӧǰ��������ʵ���/mol | CO2 | 0 | 0 | 0.2 | 0.2 |
| CO | 3.0 | 3.0 | 2.8 | 2.8 | |
| H2 | 7.0 | 7.0 | 7.0 | 7.0 | |
| ƽ��ʱCH3OH���������/�� | 4.9 | 8.8 | 36.5 | 50.7 | |
B����������ѹǿ����ʱ����Ӧ�ﵽƽ��
C��CH3OH���������ԭ����CO2���˴�����
D������CO2�����ƽ��ʱCH3OH���������
��ӦCO(g)+2H2(g)  2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
2CH3OH(g)�ں����ܱ������н��С�Ϊ̽���¶ȡ�CO2�����ضԸ÷�Ӧ��Ӱ�죬������4��ʵ�飬��������±�������˵������ȷ����
|
����� |
1 |
2 |
3 |
4 |
|
|
��Ӧ�¶�/�� |
225 |
235 |
225 |
235 |
|
|
��Ӧǰ��������ʵ���/mol |
CO2 |
0 |
0 |
0.2 |
0.2 |
|
CO |
3.0 |
3.0 |
2.8 |
2.8 |
|
|
H2 |
7.0 |
7.0 |
7.0 |
7.0 |
|
|
ƽ��ʱCH3OH���������/�� |
4.9 |
8.8 |
36.5 |
50.7 |
A���÷�Ӧ�ġ�H>0
B����������ѹǿ����ʱ����Ӧ�ﵽƽ��
C��CH3OH���������ԭ����CO2���˴�����
D������CO2�����ƽ��ʱCH3OH���������
 ��֪���淴Ӧ��FeO��s��+CO��g��
��֪���淴Ӧ��FeO��s��+CO��g�� Fe��s��+CO2��g����������ҵ�е�һ����Ҫ��Ӧ�����¶�T��n��CO2��/n��CO���ı�ֵ��ϵ�����±���ʾ�������й�˵����ȷ���ǣ�������
Fe��s��+CO2��g����������ҵ�е�һ����Ҫ��Ӧ�����¶�T��n��CO2��/n��CO���ı�ֵ��ϵ�����±���ʾ�������й�˵����ȷ���ǣ�������