ЬтФПФкШн
(8Зж)вбжЊЃКCH3CH2OH+NaBr+H2SO4(ХЈ)  CH3CH2Br+NaHSO4 +H2OЁЃ
CH3CH2Br+NaHSO4 +H2OЁЃ
ЪЕбщЪвжЦБИфхввЭщЃЈЗаЕуЮЊ38.4ЁцЃЉЕФзАжУКЭВНжшШчЯТЃК
ЂйАДгвЭМЫљЪОСЌНгвЧЦїЃЌМьВщзАжУЕФЦјУмадЃЌШЛКѓЯђUаЮЙмКЭДѓЩеБРяМгШыБљЫЎЃЛЂкдкдВЕзЩеЦПжаМгШы10mL95ЃЅввДМЁЂ28mLХЈСђЫсЃЌШЛКѓМгШыбаЯИЕФ13gфхЛЏФЦКЭМИСЃЫщДЩЦЌЃЛЂлаЁЛ№МгШШЃЌЪЙЦфГфЗжЗДгІЁЃ
ЪдЛиД№ЯТСаЮЪЬтЃК
(1)ЗДгІЪБШєЮТЖШЙ§ИпПЩПДЕНгаКьзиЩЋЦјЬхВњЩњЃЌИУЦјЬхЕФЛЏбЇЪНЮЊ
ЁЃ
ЃЈ2ЃЉЮЊСЫИќКУЕФПижЦЗДгІЮТЖШЃЌГ§гУЭМЪОЕФаЁЛ№МгШШЃЌИќКУЕФМгШШЗНЪНЪЧ__________ЁЃ
(3)ЗДгІНсЪјКѓЃЌUаЮЙмжаДжжЦЕФфхввЭщГЪзиЛЦЩЋЁЃНЋUаЮЙмжаЕФЛьКЯЮяЕЙШыЗжвКТЉЖЗжаЃЌОВжУЃЌД§вКЬхЗжВуКѓЃЌЗжвКЃЌШЁ (ЬюЁАЩЯВуЁБЛђЁАЯТВуЁБ)вКЬхЁЃЮЊСЫГ§ШЅЦфжаЕФдгжЪЃЌПЩбЁдёЯТСаЪдМСжаЕФ (ЬюађКХ)ЁЃ
| AЃЎNa2SO3ШмвК | BЃЎH2O | CЃЎNaOHШмвК | DЃЎCCl4 |
(4)ЯТСаМИЯюЪЕбщВНжшЃЌПЩгУгкМьбщфхввЭщжаЕФфхдЊЫиЃЌЦфе§ШЗЕФВйзїЫГађЪЧЃКШЁЩйСПфхввЭщЃЌШЛКѓ (ЬюађКХ)ЁЃ
ЂйМгШШ ЂкМгШыAgNO3ШмвК ЂлМгШыЯЁHNO3ЫсЛЏ ЂмМгШыNaOHШмвК ЂнРфШД
ЃЈ7ЗжЃЉЃЈ1ЃЉBr2 ЃЈ2ЃЉЫЎдЁМгШШ
ЃЈ3ЃЉЯТВуЃЌA ЃЈ4ЃЉеєСѓ
ЃЈ5ЃЉЂмЂйЂнЂлЂкЃЈ2ЗжЃЉ
НтЮі



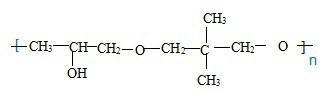
 ЃЌ
ЃЌ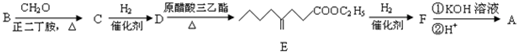

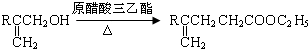


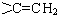 ЕФЭЌЗжвьЙЙЬхга
ЕФЭЌЗжвьЙЙЬхга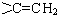 ЃЌКЫДХЙВеёЧтЦзга3ИіЗхЃЌЧвЗхУцЛ§жЎБШЮЊ9ЃК2ЃК1ЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ
ЃЌКЫДХЙВеёЧтЦзга3ИіЗхЃЌЧвЗхУцЛ§жЎБШЮЊ9ЃК2ЃК1ЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ



 ЃЈRЁЂR'ДњБэЬўЛљЛђЧтдзгЃЉ
ЃЈRЁЂR'ДњБэЬўЛљЛђЧтдзгЃЉ



 ЃЉЕФКЯГЩТЗЯпЃК
ЃЉЕФКЯГЩТЗЯпЃК


 ЁЂ
ЁЂ

