��Ŀ����
��X��Y��Z����Ԫ�أ���֪��
��X2-��Y-����Y����̬�⻯����Ӿ�����ͬ�ĵ�������
��Z��Y����ɻ�����ZY3��ZY3��Һ��KSCN��Һ�ʺ�ɫ��
��ش��������⣺
��1��Y������������Ӧˮ����Ļ�ѧʽ��__________��
��2����ZY3��Һ�����ˮ�ɵõ����ɫҺ�壬��Ӧ�����ӷ���ʽ��____________________��
��Һ����е�������__________������ĸ����
a������ͨ����Һ��ʱ�γɹ����ġ�ͨ·��
b������缫ֱͨ�������һ������Һ����ɫ����
c�����Һ���м�����������Һ����������
d������Һ����ȡ����ɡ����պ�������������
��3��X�����ڿ�����ȼ������һ����ɫ�д̼�����ζ�����塣
����֪һ�������£�ÿ1mol�����屻O2��������98.0kJ����2mol��������1mol O2�ڴ������·�����Ӧ���ﵽƽ��ʱ�ų���������176.4kJ����������ת����Ϊ__________��
��ԭ��ɫ�д̼�����ζ�������뺬1.5mol Y��һ�ֺ����ᣨ�����ij�γ�����ʵ������ȡ����������Һ��һ�������·�Ӧ��������һ��ǿ���һ�����������1.5��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
��X2-��Y-����Y����̬�⻯����Ӿ�����ͬ�ĵ�������
��Z��Y����ɻ�����ZY3��ZY3��Һ��KSCN��Һ�ʺ�ɫ��
��ش��������⣺
��1��Y������������Ӧˮ����Ļ�ѧʽ��__________��
��2����ZY3��Һ�����ˮ�ɵõ����ɫҺ�壬��Ӧ�����ӷ���ʽ��____________________��
��Һ����е�������__________������ĸ����
a������ͨ����Һ��ʱ�γɹ����ġ�ͨ·��
b������缫ֱͨ�������һ������Һ����ɫ����
c�����Һ���м�����������Һ����������
d������Һ����ȡ����ɡ����պ�������������
��3��X�����ڿ�����ȼ������һ����ɫ�д̼�����ζ�����塣
����֪һ�������£�ÿ1mol�����屻O2��������98.0kJ����2mol��������1mol O2�ڴ������·�����Ӧ���ﵽƽ��ʱ�ų���������176.4kJ����������ת����Ϊ__________��
��ԭ��ɫ�д̼�����ζ�������뺬1.5mol Y��һ�ֺ����ᣨ�����ij�γ�����ʵ������ȡ����������Һ��һ�������·�Ӧ��������һ��ǿ���һ�����������1.5��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
��1��HClO4
��2��Fe3++3H2O Fe(OH)3(����)+3H+��abd
Fe(OH)3(����)+3H+��abd
��3����90% ��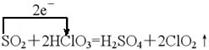
��2��Fe3++3H2O
 Fe(OH)3(����)+3H+��abd
Fe(OH)3(����)+3H+��abd��3����90% ��
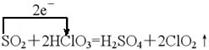
����ΪԪ���ƶ��⣬���ھ������͡�
��1����ZY3��KSCN��Һ�ʺ�ɫ��֪ZY3ΪFeCl3��YΪ��Ԫ�أ���X2-��Y-��HCl������ͬ�ĵ�������18������֪XΪ��Ԫ�أ�
��2��FeCl3�����ˮ������Fe(OH)3���壬�ܷ������������Ӿ��;
��3����S�ڿ�����ȼ������SO2����O2��һ��������2SO2��O2 2SO3
2SO3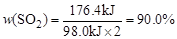 �����ɸ����ij�γ�����ʵ������O2���Ƴ�Y�ĺ�����ΪHClO3��1.5mol HClO3��Ӧת��1.5mol e-֪ClԪ�ػ��ϼ۽��ͼ���ԭ����ΪClO2��S�������ɣ�6�ۡ�
�����ɸ����ij�γ�����ʵ������O2���Ƴ�Y�ĺ�����ΪHClO3��1.5mol HClO3��Ӧת��1.5mol e-֪ClԪ�ػ��ϼ۽��ͼ���ԭ����ΪClO2��S�������ɣ�6�ۡ�
��1����ZY3��KSCN��Һ�ʺ�ɫ��֪ZY3ΪFeCl3��YΪ��Ԫ�أ���X2-��Y-��HCl������ͬ�ĵ�������18������֪XΪ��Ԫ�أ�
��2��FeCl3�����ˮ������Fe(OH)3���壬�ܷ������������Ӿ��;
��3����S�ڿ�����ȼ������SO2����O2��һ��������2SO2��O2
 2SO3
2SO3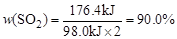 �����ɸ����ij�γ�����ʵ������O2���Ƴ�Y�ĺ�����ΪHClO3��1.5mol HClO3��Ӧת��1.5mol e-֪ClԪ�ػ��ϼ۽��ͼ���ԭ����ΪClO2��S�������ɣ�6�ۡ�
�����ɸ����ij�γ�����ʵ������O2���Ƴ�Y�ĺ�����ΪHClO3��1.5mol HClO3��Ӧת��1.5mol e-֪ClԪ�ػ��ϼ۽��ͼ���ԭ����ΪClO2��S�������ɣ�6�ۡ�
��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д�
�����Ŀ
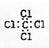


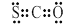 �ڶ��������ӵĽṹ��ʽ��
�ڶ��������ӵĽṹ��ʽ��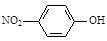
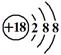 �ܼ�����ӵı���ģ�ͣ�
�ܼ�����ӵı���ģ�ͣ�
 CO32����H3O��
CO32����H3O�� Cu + H2O
Cu + H2O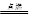 2NaCl
2NaCl 2H2O + O2��
2H2O + O2��