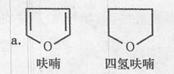
��Ŀ����
��15�֣��ڼ���ڵ������£�±�����봼��Ӧ�����ѣ�R-O-R��):

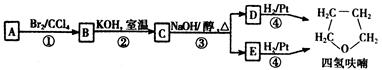
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ���£�

��ش��������⣺
��1��1 mol A��1 mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ __________________________
A���������������ŵ������� __________________________
A�Ľṹ��ʽΪ__________________________��
��2���ڢ٢ڲ���Ӧ���ͷֱ�Ϊ�� _____________ �� _____________��
��3��������B���еĻ�ѧ���ʣ���д��ĸ���ţ��� _____________��
a.�ɷ���������Ӧ b.ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c.�ɷ����ӳɷ�Ӧ d.�������¿ɷ����Ӿ۷�Ӧ[��Դ:ѧ,��,��]
��4��д��C��D��E�Ľṹ��ʽ��
C _____________ D ___________________E_____________
��5��д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��
_____________________________________________________________________________
��6��д���������״���������ͬ���칹��Ľṹ��ʽ��
_____________________________________________________________________________
____________________________________________________________________________
��1��C4H10O �ǻ���̼̼˫�� CH2 = CHCH2CH2��OH
��2���ӳ� ȡ����3��ab��4��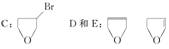
��5��
��6��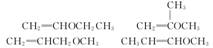
��������