��Ŀ����
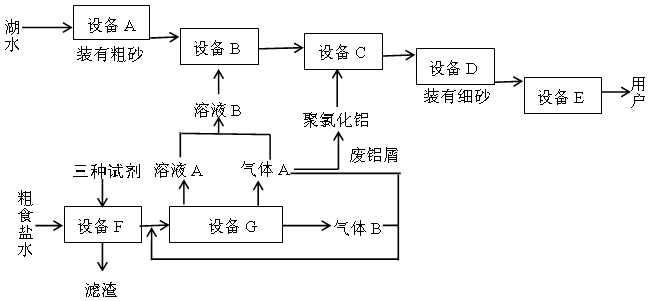
��ҵ�ϵ�ⱥ��ʳ��ˮ����ȡ���ֻ���ԭ�ϣ����в���ԭ�Ͽ������Ʊ��ྦྷ�衣
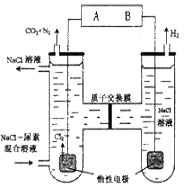
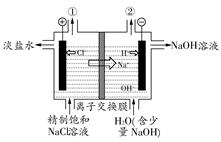
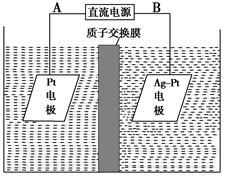
(1)��ͼ�����ӽ���Ĥ����ⱥ��ʳ��ˮʾ��ͼ����������������������________��NaOH��Һ�ij���Ϊ________(����ĸ)�����Ʊ���ʳ��ˮ�Ľ���Ϊ________(����ĸ)����������Ӧʹ�õ�Һ����________��
(2)�ྦྷ����Ҫ����SiHCl3��ԭ�����������丱����SiCl4���ۺ������ܵ��㷺��ע��
��SiCl4���������̿��(����ά��Ҫԭ����ͬ)������Ϊ������SiCl4��H2��O2��Ӧ�����������֣���ѧ����ʽΪ___________________________________��
��SiCl4��ת��ΪSiHCl3��ѭ��ʹ�ã�һ�������£���20 L�����ܱ������еķ�Ӧ��
3SiCl4(g)��2H2(g)��Si(s) 4SiHCl3(g)
4SiHCl3(g)
��ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140 mol/L��0.020 mol/L����H2ȫ����Դ�����ӽ���Ĥ���ĵ���������������Ĵ�NaCl������Ϊ________kg��
(3)������Ĥ���۵�ⱥ��ʳ��ˮ������ȡ�����ƣ�ͬʱ�������������Ƶ�������213.0 kg������������________m3(��״��)��

(1)��ͼ�����ӽ���Ĥ����ⱥ��ʳ��ˮʾ��ͼ����������������������________��NaOH��Һ�ij���Ϊ________(����ĸ)�����Ʊ���ʳ��ˮ�Ľ���Ϊ________(����ĸ)����������Ӧʹ�õ�Һ����________��
(2)�ྦྷ����Ҫ����SiHCl3��ԭ�����������丱����SiCl4���ۺ������ܵ��㷺��ע��
��SiCl4���������̿��(����ά��Ҫԭ����ͬ)������Ϊ������SiCl4��H2��O2��Ӧ�����������֣���ѧ����ʽΪ___________________________________��
��SiCl4��ת��ΪSiHCl3��ѭ��ʹ�ã�һ�������£���20 L�����ܱ������еķ�Ӧ��
3SiCl4(g)��2H2(g)��Si(s)
 4SiHCl3(g)
4SiHCl3(g)��ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140 mol/L��0.020 mol/L����H2ȫ����Դ�����ӽ���Ĥ���ĵ���������������Ĵ�NaCl������Ϊ________kg��
(3)������Ĥ���۵�ⱥ��ʳ��ˮ������ȡ�����ƣ�ͬʱ�������������Ƶ�������213.0 kg������������________m3(��״��)��
(1)������a��d��Ũ���ᡡ(2)��SiCl4��2H2��O2 SiO2��4HCl����0.35��(3)134.4
SiO2��4HCl����0.35��(3)134.4
 SiO2��4HCl����0.35��(3)134.4
SiO2��4HCl����0.35��(3)134.4(1)��ⱥ��ʳ��ˮ�ĵ缫��Ӧ�ֱ�Ϊ��������2H����2e��=H2����������2Cl����2e��=Cl2����������������Ϊ������H���������ŵ磬ˮ�е�c(OH��)����NaOH������(a��)��������ⱥ��ʳ��ˮ���õ������ӽ���Ĥֻ����������(Na��)ͨ������ֹ�����Ӻ�����ͨ�����������У�Cl���������������ģ����Ӧ��������(d��)����NaCl���������������ΪCl2��Ӧ��ŨH2SO4��
(2)�ٹ��άΪSiO2������Ϊ���֣���һ������ֻ��ΪHCl��
��n(H2)��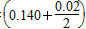 ��20��3 mol��
��20��3 mol��
��2NaCl��2H2O 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��
��2��58.5������������������ 2
��m(NaCl)���������������� 6 g
m(NaCl)��351 g��0.35 kg��
(3)�����⣬��д����Ӧ�Ļ�ѧ����ʽΪ��
NaCl��3H2O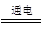 NaClO3��3H2��
NaClO3��3H2��
������������ 106.5����6
������������ 213 kg��m(H2)
m(H2)��12 kg��n(H2)��6 000 mol��V(H2)��134.4 m3��
(2)�ٹ��άΪSiO2������Ϊ���֣���һ������ֻ��ΪHCl��
��n(H2)��
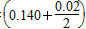 ��20��3 mol��
��20��3 mol����2NaCl��2H2O
 2NaOH��H2����Cl2��
2NaOH��H2����Cl2����2��58.5������������������ 2
��m(NaCl)���������������� 6 g
m(NaCl)��351 g��0.35 kg��
(3)�����⣬��д����Ӧ�Ļ�ѧ����ʽΪ��
NaCl��3H2O
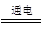 NaClO3��3H2��
NaClO3��3H2�������������� 106.5����6
������������ 213 kg��m(H2)
m(H2)��12 kg��n(H2)��6 000 mol��V(H2)��134.4 m3��

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ
 Cl2����H2��
Cl2����H2��
 CuSO4��H2��
CuSO4��H2��