��Ŀ����
��14�֣��ζ����ǻ�ѧ�о��г��õĶ���ʵ�鷽����
��ij��ѧ��ȤС������֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�����������Һ�ⶨ��Ũ�ȡ�
��ʵ����Ӧѡ�õ�ָʾ��Ϊ ��
�����в������²ⶨ���ƫ�ߵ��� ��
a����ʽ�ζ���������ˮ��ϴ��δ��������ϴ
b����ƿ������ˮ��ϴ��δ�ô�������������Һ��ϴ
c����ʽ�ζ��ܵζ�ǰ���촦�����ݣ��ζ���������ʧ
��Ī�ַ���һ�ֳ����ζ���.�ⶨij��Һ�ĵ�c(Cl��),��K2CrO4Ϊָʾ�����ñ���������Һ�ζ�����Һ[Ksp(AgCl)=1.56��10��10, Ksp(Ag2CrO4)=1.10��10��12,Ag2CrO4Ϊש��ɫ]
�ٵζ��յ�������� ��
�ڸõζ����˵�pH��Χ��6.5��10.5������Һ������δ��ڣ�c(NH4+)��0.05mol/Lʱ��Ӧ����Һ��pH������6.5��7.2���������й�˵������Ϊ��ȷ���� ��
a������ҺpH��6.5����ƽ��Cr2O72��+H2O 2CrO42��+2H+���ƣ����µζ��յ��ͺ�
2CrO42��+2H+���ƣ����µζ��յ��ͺ�
b������Һ������δ��ڣ���pH��7.2ʱ�������������[Ag(NH3)2]+�������յ��ͺ�
c���ζ�ʱӦ����ҡ������ʹ��AgCl����������Cl����ʱ�ͷų�������ֹ�ζ��յ��ͺ�
��������ԭ�ζ���ˮ�������ij��÷��������ڲⶨ��ˮ�еĻ�ѧ����������λmg/L����ÿ��ˮ���л�ԭ�����ʱ�������O2����������ij��ȤС��ÿ��ȡ100mL��ˮ���������ữ����0.01667mol/LK2CrO7��Һ25.00mL��ʹˮ���еĻ�ԭ��������ȫ������Ȼ����0.1000mol/LFeSO4����Һ�ζ�ʣ���Cr2O72����ʵ�����ݼ�¼���£�
|
ʵ����� |
FeSO4��Һ���������/mL |
|
|
�ζ�ǰ |
��� |
|
|
1 |
0.10 |
16.20 |
|
2 |
0.30 |
15.31 |
|
3 |
0.20 |
15.19 |
�Իش��������⣺
��___Cr2O72��+____Fe2++____ ________==_____Cr3++_____Fe3++____H2O
�ڼ���÷�ˮ�Ļ�ѧ����������д��������̣��������һλС������
�Ţٷ�̪�����ȣ���2�֣���a��c��2�֣� �Ƣ�����ש��ɫ������2�֣���a��b��2�֣�
�Ǣ� Cr2O72��+ 6 Fe2++ 14 H+ == 2 Cr3++ 6 Fe3++ 7 H2O��2�֣� ��80.0mg/L��4�֣�
����������1����ǿ���ǿ��֮��ĵζ�����̪����Ⱦ�����ָʾ����
��a�൱��ϡ������ᣬ��������������ƫ�ⶨ���ƫ�ߣ�b����ȷ�IJ�������Ӱ�죻c�൱��������������ƫ�ⶨ���ƫ�ߣ���ѡac��
��2���ٵ�������ǡ�÷�Ӧ֮���ټ�����������������ɸ�����ש��ɫ�������ݴ˿��ж��յ㡣
�ڵζ�ʱ����ҡ��������ʹ��Һ�Ž����������ʵ��������c�Ǵ���ġ��������ȷ�ġ���ѡab��
��3���ٸ���������ԭ��Ӧ�е��ӵĵ�ʧ�غ������ƽ����Ԫ�صĻ��ϼ��ɣ�6�۽��͵���3�ۣ�����1mol�������õ�6mol���ӡ���1mol��ԭ����������ֻ��ʧȥ1mol���ӣ������������ͻ�ԭ�������ʵ���֮����1�U6����ʽΪCr2O72��+ 6 Fe2++ 14 H+ == 2 Cr3++ 6 Fe3++ 7 H2O��
������ʵ�����ĵĵ�����������Һ����ֱ���16.10ml��15.01ml��14.99ml����˵����һ��ʵ����ʧ�ܵġ�ȡ�����ε����ƽ��ֵΪ15.00ml��������K2CrO7�����ʵ�����2.5��10��4mol����˺ͻ�ԭ�����ʷ�Ӧ��K2CrO7�����ʵ�����4.1675��10��4mol��2.5��10��4mol��1.6675��10��4mol��ת�Ƶ�����1.6675��10��4mol��6��1.0��10��3mo��������Ҫ������1.0��10��3mo��4��2.5��10��4mol�����Է�ˮ�еĻ�ѧ��������2.5��10��4mol��32g/mol��1000mg/g��0.1L��80.0mg/L��


 ��
��
 )������ˮ��ClO2��
)������ˮ��ClO2��
 ������4�м����ָʾ��Ϊ_________���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ___________________________��
������4�м����ָʾ��Ϊ_________���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ___________________________�� ����Һ�е�
����Һ�е� ��ԭΪ
��ԭΪ �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ_________________
_____________��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ_________________
_____________��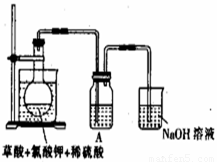
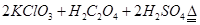
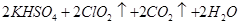
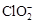 )������ˮ��ClO2��
)������ˮ��ClO2��
 ������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
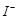 ����Һ�е�
����Һ�е�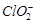 ��ԭΪ
��ԭΪ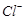 �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��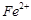 ��
��


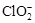 )������ˮ��ClO2��
)������ˮ��ClO2��
 ������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ
���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
 ����Һ�е�
����Һ�е� ��ԭΪ
��ԭΪ �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ�� ��
��