��Ŀ����
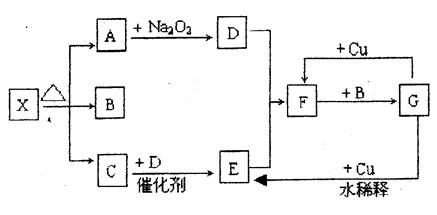
��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�����CΪO2��DΪCl2��EΪFe���ʣ�����Ϊ��������Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��

��1��д���й����ʵ����ƻ�ѧʽ��
B_______________��F__________________��H____________________��
��2��ָ��MnO2����ط�Ӧ�е����ã���Ӧ������_________������Ӧ������__________����
��3������Ӧ�����ڼ��������½��У���A��_____________������Ӧ�����ڳ��������½��У���A��_____________��
��4��д��B��MnO2���Ȼ��D�Ļ�ѧ����ʽ��__________________________________��

��1��д���й����ʵ����ƻ�ѧʽ��
B_______________��F__________________��H____________________��
��2��ָ��MnO2����ط�Ӧ�е����ã���Ӧ������_________������Ӧ������__________����
��3������Ӧ�����ڼ��������½��У���A��_____________������Ӧ�����ڳ��������½��У���A��_____________��
��4��д��B��MnO2���Ȼ��D�Ļ�ѧ����ʽ��__________________________________��
(1)ŨHCl Fe3O4 FeCl3 (2)�� ���� (3)KClO3 H2O2
(4) 4HCl+MnO2 MnCl2+H2O+Cl2
MnCl2+H2O+Cl2
(4) 4HCl+MnO2
 MnCl2+H2O+Cl2
MnCl2+H2O+Cl2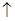
���������AΪH2O2��BΪŨ���CΪO2��DΪCl2��EΪFe��FΪFe3O4��GΪFeCl2��HΪFeCl3��
��2����Ӧ��Ϊ��������ֽ���������MnO2��Ϊ�������ӿ췴Ӧ�Ľ��С���Ӧ���У�MnO2��Ϊ����������Ũ���ᷴӦ�Ʊ�������
��3��CΪO2��ʵ�����Ʊ������ķ���ʽ���漰��MnO2���м���KClO3�ֽ���������H2O2�ֽ���������
��4����ѧ����ʽΪ 4HCl+MnO2
 MnCl2+H2O+Cl2
MnCl2+H2O+Cl2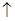
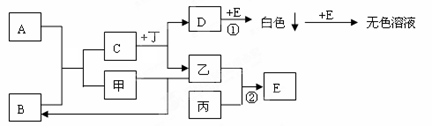
���������ƶ���������ۺϿ������������Ʊ��Լ��������Ʊ��������е��⡣���ƶ���Ľ��Ӧע��Ѱ��ͻ�ƿڡ�

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д� �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ
 ��46����
��46����