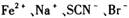��Ŀ����
����������������Һ���й���������ȷ����
| | �� | �� | �� | �� |
| Ũ��c/mol/L | 0.1 | 0.1 | 0.1 | 0.1 |
| ��Һ | ��ˮ | CH3COONa��Һ | ���� | ���� |
| A����20mL����Һ����μ������Һ����Һ���������仯����ͼ��1����ʾ |
| B���ڡ�������Һ�������ϣ�����Ũ�ȣ�2c(Na+)=c(CH3COO-)+c(CH3COOH) |
C���â۵ζ��٣��÷�̪��ָʾ�����ζ���������ͼ��2����ʾ��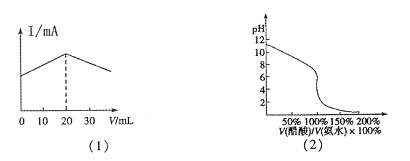 |
| D���١�������Һ�������ϣ�����Ũ�ȣ�c(Cl-)>c(NH4+)>c(H+)>c(OH-) |
C
��

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ
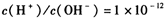 ����Һ��
����Һ��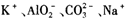
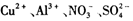
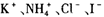
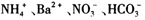
 .24��(��״̬)����ͨ��250mLŨ��Ϊ0.1mol��L��1��������Һ�У���ַ�Ӧ����Һ�и�����Ũ���ɴ�С��˳��Ϊ������
.24��(��״̬)����ͨ��250mLŨ��Ϊ0.1mol��L��1��������Һ�У���ַ�Ӧ����Һ�и�����Ũ���ɴ�С��˳��Ϊ������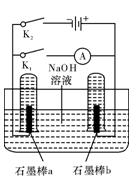
 ·���е���ͨ����д��a���ĵ缫��Ӧʽ ����
·���е���ͨ����д��a���ĵ缫��Ӧʽ ����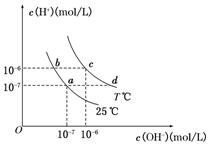



 ��
��
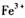 ����Һ�У�
����Һ�У�