��Ŀ����
����Ϊ�����ϸ�������������ṩ�����������ǣ����Ź�ҵ�ͽ�ͨ����Ѹ�ٷ�չ�������˿ڸ߶ȼ��У�����������д����ŷ��̳����к�����ȣ����ڶԴ��������Ⱦ���ҹ�ij��ҵ���еġ����������ձ�����ʾ���ó��еĿ����ܵ�һ���̶ȵ���Ⱦ��ij�о���ѧϰС��Ըó��еĿ�����Ⱦ�����������о���(1)С����һͬѧ���������ó����ó��п�����Ⱦ����Ҫԭ����A.ʹ��ʯ��Һ����B.ȼ�պ���úC.�۳���Ⱦ��������һͬѧ��Ϊ����һ�������ų���ԭ���ǣ�D.__________________��
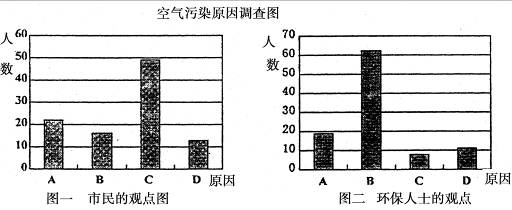
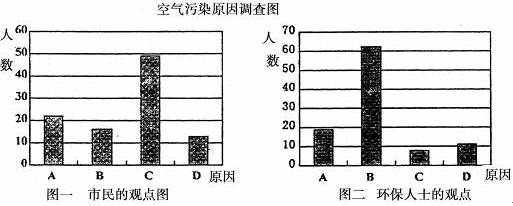
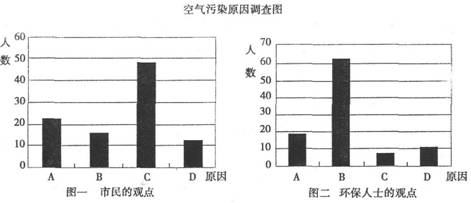
�о���ѧϰС�����ɸó��п�����Ⱦ������Ҫԭ������˸���100�������100λ����������ʿ������������ͼ��ʾ��
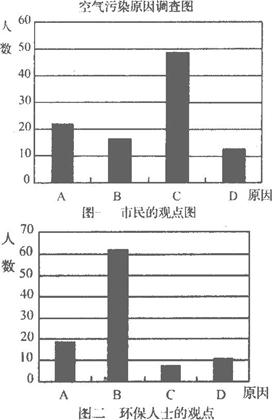
�ӻ�����ʿ�Ĺ۵��Ϸ���������Ϊ��ɸó��п�����Ⱦ����Ҫ�к��ɷ���____________(�û�ѧʽ��ʾ)��
(2)������Ⱦ���γ����ꡣ�о���ѧϰС��Ըó��е���ˮ�����˲�����������ղɼ�ʱ���pHΪ4.82�������ձ��о�2h���ٴβ��pHΪ4.68���Դˣ���ĺ���������___________________��
(3)�о���ѧϰС��ͬѧȡ����(��������������������̼�����������)10.0L(������ɱ�״��)������ͨ��������ˮ����������Һ�м���������Ȼ�����Һ��������ɫ������������ϴ�ӡ�����Ƶ�������Ϊ0.023 3 g���Իش�ð�ɫ�����Ļ�ѧʽΪ____________��ͨ������øÿ�������Ҫ�к�����ɷֵ��������Ϊ____________��
(4)��С��ͬѧ������д�ʩ�Լ��ٿ�����Ⱦ����Ҫ�к��ɷ��ŷ���������Ϊ��������____________(�����)��
������Ȼ������ú̿������ȼ�� �ڸĽ�ȼú����������ú������ �۹�������ʱ��ȼú��¯���̴���ø��� ��ȼú�м�������ʯ��ʯ��ʹ��
(1)������β����Ⱦ SO2
(2)��ˮ��Ʒ�е�H2SO3�������е�����������H2SO4
(3)BaSO4 0.022 4��
(4)�٢ڢ�
���������⿼��ѧ���Ի�������֪ʶ���˽⣬�����ʣ�
SO2+Br2+2H2O====2HBr+H2SO4
H2SO4+BaCl2====BaSO4��+2HCl
�����ù�ϵʽ��
SO2��BaSO4
1 mol 233g
xmol 0.023 3 x=0.000 1 mol
![]() ��l00��=0.022 4��
��l00��=0.022 4��

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�