��Ŀ����
ʵ������MnO2��Ũ���������ȡ�������������������ͼ��ʾ��
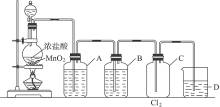
(1)��ȡCl2�Ļ�ѧ��Ӧ����ʽ______________________��
(2)Aƿ��Һ����___________��������___________��ԭ��___________��Bƿ��Һ����___________��������___________��Cƿ��ȡ___________�ſ�����������ΪCl2�ܶ�___________������D�ձ���Һ��ijɷ���___________����������___________��������Ӧ�ķ���ʽ___________��
(3)ʹ�÷�Һ©���μ�Ũ���ᣬ�����÷�Һ©���л�������___________��___________�����û�з�Һ©����ֻ�г���©����С�Թܣ���___________(����ԡ������ԡ�)��?������
(1)4HCl(Ũ)+MnO2![]() MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
(2)����ʳ��ˮ ����Cl2�е�HCl���� Cl2�ڱ���ʳ��ˮ���ܽ��С��HCl��������ʳ��ˮ������ȥ Ũ���� ����Cl2�е�ˮ�� ���� ���� NaOH ���ն�����ж�Cl2Cl2+2NaOH====NaCl+NaClO+H2O
(3)����HCl���� ���ܷ������ ����
����:
�������ۺϿ���Cl2��ʵ�����Ʊ���Ӧ�������������ռ���β�������գ�����������������ǿ���������ܵĿ��ء�

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
