��Ŀ����
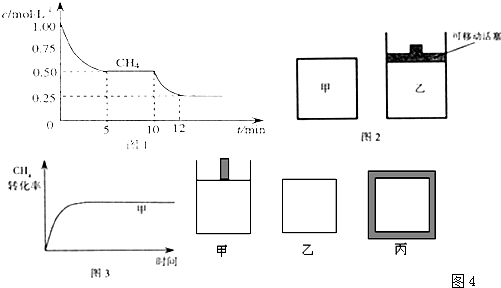
 ��һ�ܱ������з�����Ӧ��2X��g��+Y��g��?aZ��g����H=QkJ?mol-1����ʼ����� ��2��1��X��Y���뷴Ӧ���У�һ�������·�����Ӧ����ͼ1��ͼ2�Ǹ��ݷ�Ӧ���Ƶ�ͼ�������й�˵����ȷ���ǣ�������
��һ�ܱ������з�����Ӧ��2X��g��+Y��g��?aZ��g����H=QkJ?mol-1����ʼ����� ��2��1��X��Y���뷴Ӧ���У�һ�������·�����Ӧ����ͼ1��ͼ2�Ǹ��ݷ�Ӧ���Ƶ�ͼ�������й�˵����ȷ���ǣ�������| A��ͼ1��P1��P2��a��3 | B��ͼ1��T1��T2��Q��0 | C��ͼ2��t1ʱ��ʾ���¡���ѹ�����£���ƽ����ϵ�г���һ������Z���� | D��ͼ2�������=3��t2ʱ��ʾ��������ϵ�м����˴��� |
B����P2T1��P2T1��֪��T2�ȴﵽƽ�⣬��T1��T2���¶ȸߣ���Z���������С���������¶ȣ�ƽ�������ƶ�������Q��0����B����
C��ͼ2��t1ʱ�淴Ӧ������������Ӧ���ʼ�С��������������Ũ�ȵ�ͬʱ���ٷ�Ӧ��Ũ�ȣ�ֻ����Z��ͼ������C����
D�������=3���÷�ӦΪ�����������ķ�Ӧ��t2ʱ���淴Ӧ����ͬ�ȳ̶ȵ�������t2ʱ��ʾ����ϵ�м����˴���������ѹǿ����D��ȷ��
��ѡD��

������ҵ���ҹ������д���ʮ����Ҫ��λ�ã���ҵ�ϲ��ø�¯ұ�������ó�����̿���������ۼ���ʯ��ʯ����ԭ�ϡ���֪������ԭ����ԭʱ�����еģ���ԭʱ�¶ȼ�CO��CO2ƽ����������CO����������Ĺ�ϵ����ͼ��

��1������Ԫ�����ڱ���λ�� ���� ��
��2�����¶ȵ���570��ʱ����ԭ��������ɣ����¶ȸ���570��ʱ�����η����Ļ�ԭ��Ӧ�У� ��ѡ����ͼ�е�a��b��c��d��գ�
��3��Ϊ���ٸ�¯ұ��ʱ����CO��β���ŷţ������о�����ȡ���� ��
��a�������������䣬���Ӹ�¯�ĸ߶�
��b�����ڻ�ԭʱ��¯��
��c������ԭ���н�̿�������ı���
��d�������ɵ���ˮ��ʱ�Ƴ�
��4����֪���з�Ӧ��ֵ��
|
��Ӧ��� |
��ѧ��Ӧ |
��Ӧ�� |
|
�� |
Fe2O3(s)��3CO(g)=2Fe(s)��3CO 2(g) |
��H1= -26.7kJ��mol-1 |
|
�� |
3Fe2O3(s)��CO(g)=2Fe3O4(s)��CO2(g) |
��H2= -50.8kJ��mol-1 |
|
�� |
Fe3O4(s)��CO(g)=3FeO(s)��CO2 (g) |
��H3= -36.5kJ��mol-1 |
|
�� |
FeO(s)��CO(g)=Fe(s)��CO2(g) |
��H4 |
��Ӧ�ܡ�H4= kJ��mol-1��
��5��1100��ʱ�� FeO(s)��CO(g)  Fe(s)��CO2(g)��ƽ�ⳣ��K=0.4������һ�ܱ������У�����7.2gFeO��ͬʱͨ��4.48LCO(���ۺ�Ϊ��״��)���������µ�1100�棬��ά���¶Ȳ��䣬��ƽ��ʱ��FeO��ת����Ϊ��
��
Fe(s)��CO2(g)��ƽ�ⳣ��K=0.4������һ�ܱ������У�����7.2gFeO��ͬʱͨ��4.48LCO(���ۺ�Ϊ��״��)���������µ�1100�棬��ά���¶Ȳ��䣬��ƽ��ʱ��FeO��ת����Ϊ��
��


 ������ҵ���ҹ������д���ʮ����Ҫ��λ�ã���ҵ�ϲ��ø�¯ұ�������ó�����̿���������ۼ���ʯ��ʯ����ԭ�ϣ���֪������ԭ����ԭʱ�����еģ���ԭʱ�¶ȼ�CO��CO2ƽ����������CO����������Ĺ�ϵ��ͼ��
������ҵ���ҹ������д���ʮ����Ҫ��λ�ã���ҵ�ϲ��ø�¯ұ�������ó�����̿���������ۼ���ʯ��ʯ����ԭ�ϣ���֪������ԭ����ԭʱ�����еģ���ԭʱ�¶ȼ�CO��CO2ƽ����������CO����������Ĺ�ϵ��ͼ��