��Ŀ����
����Ŀ��A��B��C��DΪԭ�������������������Ԫ�أ�A2����B+������ͬ�ĵ��ӹ��ͣ�C�� DΪͬ����Ԫ����C�������������������������3����DԪ���������һ��δ�ɶԵ��ӡ��ش��������⣺
��1������Ԫ���е縺����С����________����Ԫ�ط��ţ�������Cԭ�ӵ���Χ�����Ų�ͼΪ________��
��2��A��B���⻯�������ľ������ͷֱ�Ϊ_________��_________��
��3��B��C��������D�γɻ���������۵�ϸߵ���____���û�ѧʽ��ʾ��
��4��A��B���γ�1:1�͵Ļ�����E��E�ĵ���ʽΪ_____
��5��������D2A�����幹��Ϊ_________������ԭ�ӵŵ��Ӷ���Ϊ_________������D��ʪ���Na2CO3��Ӧ���Ʊ�D2A���仯ѧ����ʽΪ_______________��
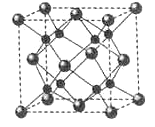
��6��A��B�ܹ��γɻ�����F���侧���ṹ��ͼ��ʾ�������߳�Ϊ0.566nm�� F �Ļ�ѧʽΪ______��������A ԭ�ӵ���λ��Ϊ______������F���ܶ�=______g��cm��3��ֻ��ʽ�������㣩
���𰸡���1��Na��1�֣��� ��1�֣�![]()
��2�����Ӿ��壨1�֣� ���Ӿ��壨1�֣�
��3��NaCl
��4��![]()
��5��V��2��
2Cl2��2Na2CO3��H2O��Cl2O��2NaHCO3��2NaCl
��6��Na2O��8��![]() ����NA��ʾҲ�ɣ�
����NA��ʾҲ�ɣ�
�����������������C�������������������������3����ӦΪPԪ�أ�C��DΪͬ����Ԫ�أ���ӦΪ��������Ԫ�أ�DԪ���������һ��δ�ɶԵ��ӣ�ӦΪClԪ�أ�A2-��B+������ͬ�ĵ��ӹ��ͣ����ԭ��������ϵ��֪AΪOԪ�أ�BΪNaԪ�أ�
��1������Ԫ�طֱ�ΪO��Na��P��Cl���縺������ΪOԪ�أ�CΪPԪ�أ���������Ų�Ϊ1s22s22p63s23p3���۵����Ų�ͼΪ![]() ��
��
��2��A���⻯��Ϊˮ��Ϊ���Ӿ��壬B���⻯��ΪNaH��Ϊ���Ӿ��壬
�ʴ�Ϊ��O3��O3��Է��������ϴ��»����ϴ��Ӿ��壻���Ӿ��壻
��3��Na��P���ܺ�Cl�γɻ�����NaCl��PCl3������NaClΪ���Ӿ��壬�۷е�ϸߣ�
��4��O��Na���γ�1:1�͵Ļ�����Na2O2�������ʽΪ![]() ��
��
��5��������D2AΪCl2O��OΪ����ԭ�ӣ��γ�2���������µ��Ӷ���Ϊ![]() =2��������ԭ�ӵļ۲���Ӷ���Ϊ4�����幹��ΪV�Σ�������ʪ���Na2CO3��Ӧ���Ʊ�Cl2O����Ӧ�ķ���ʽΪ2Cl2+2Na2CO3+H2O=Cl2O+2NaHCO3+2NaCl��
=2��������ԭ�ӵļ۲���Ӷ���Ϊ4�����幹��ΪV�Σ�������ʪ���Na2CO3��Ӧ���Ʊ�Cl2O����Ӧ�ķ���ʽΪ2Cl2+2Na2CO3+H2O=Cl2O+2NaHCO3+2NaCl��
��6��A��B�ܹ��γɻ�����FΪ���ӻ����������λ�ھ����Ķ�������ģ�������λ�ھ��������ģ���Na�ĸ���Ϊ8��O�ĸ���Ϊ8��+6��=4��N��Na����N��O��=2��1�����γɵĻ�����ΪNa2O��������Oλ�ڶ��㣬Naλ�����ģ�ÿ����������1��Na��O�ľ��������ÿ������Ϊ8���������У�����Oԭ�ӵ���λ��Ϊ8������������Ϊ![]() �����������Ϊ��0.566��10-7��cm3������F���ܶ�Ϊ
�����������Ϊ��0.566��10-7��cm3������F���ܶ�Ϊ![]() ��
��