��Ŀ����
(8��)Ϊ��̽������������Ӧ��ȡ�Ȼ�����ʵ�飬ijͬѧ����������µ�ʵ�鷽������ʵ��װ������ͼ������װ��ʡ�ԡ���֪���Ȼ���������ˮ���ۡ��е�ͣ������ױ�Ϊ��̬�����������ױ�Ϊ��̬��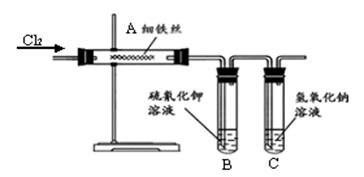
(1) �Թ�B�е�������___________________��
�Թ�B�е�������___________________��
��Ӧ�����ӷ���ʽ��____________________________ _��
(2)�Թ�C������������Һ������___________________________________��
(3)ijͬѧ�Ҳ²�����������Ӧ���ܻ��������Ȼ�������Ϊ��̽�����������Ƿ����Ȼ�����(����������ȫ��Ӧ)���������ʵ�鷽��
(4)�Ȼ���������ˮ���ۡ��е�ͣ������ױ�Ϊ��̬�����������ױ�Ϊ��̬������Ϊ��ͬѧ����ʵ��װ����ȱ�ݡ�����Ϊ��ͬѧ�жϵ������ǣ��� �� �� �� �� ��
(8��)
(1)��Һ��Ѫ��ɫ ��1���� Fe3++3SCN- = Fe(SCN)3 (2��)
(2)����δ��Ӧ���Cl2���Է���Ⱦ������(1��)
(3)ȡ������������������ �Թܼף�������������ˮ�ܽ��������KMnO4��Һ�����Ϻ�ɫ��Һ��ȥ����֤������Fe2+����(2��)
�Թܼף�������������ˮ�ܽ��������KMnO4��Һ�����Ϻ�ɫ��Һ��ȥ����֤������Fe2+����(2��)
(4)ͨ��KSCN��Һ���ܿھ�̫С����������( ������������������������𰸾�����)(2��)
����

