��Ŀ����
��9�֣�X��Y��Z��W��Ϊ��ѧ��ѧ�ij������ʣ�һ������������֮��������ת����ϵ��
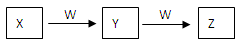
��1�����÷�Ӧ�Ƿ�������ԭ��Ӧ
��XΪǿ����Һ��W���γ��������Ҫ���壬��Y��Z�����ӷ���ʽΪ ��
��X��һ���Σ���μ���W����Һ�п�ʼ�����ݣ��������ɫ��ζ����Z����Z�ĵ���ʽΪ ��
��2�����÷�Ӧ��������ԭ��Ӧ
��X������Ϊ����ɫ���壬W��������Ӧ����㷺�Ľ���֮һ����Y��ˮ��Һ�м���W����Z��Һ�����ӷ���ʽΪ ��
��X������Ϊ���廯���Z��һ�ֺ���ɫ���壬X����ļ��鷽�� ��Xת��ΪY�Ļ�ѧ����ʽΪ ��
��1����SO32-+SO2+
H2O��HSO3-��2�֣� ��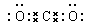 ��2�֣�
��2�֣�
��2����2Fe3+ + Fe = 3Fe2+ ��2�֣�
��ʪ��ĺ�ɫʯ����ֽ���� ��1�֣� 4NH3+5O2=4NO+6H2O��2�֣�
����������1���γ��������Ҫ������SO2������Y���������Σ��������μ�������SO2��������Ӧ����ʽ�Σ��������ӷ���ʽΪSO32-+SO2+
H2O��HSO3-��̼���κ��ᷴӦ��ʼ�β��������壬��̼������ȫת��Ϊ̼�����κ��ٵ�����ſ�ʼ����CO2���塣CO2���ɼ��Լ��γɵĹ��ۻ���������ʽΪ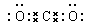 ��
��
��2������ɫ������������Ӧ����㷺�Ľ�����������Y���Ȼ������Ȼ������������������Ȼ�����������ʽΪ2Fe3+ + Fe = 3Fe2+������ɫ������NO2����Y��NO��W��������X�ǰ������������ð����Ǽ���������м��飬��������������������NO������ʽΪ4NH3+5O2=4NO+6H2O��

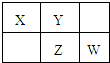 X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е�λ����ͼ��ʾ����Yԭ�ӵ������������Ǵ�����������3��������˵����ȷ���ǣ�������
X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е�λ����ͼ��ʾ����Yԭ�ӵ������������Ǵ�����������3��������˵����ȷ���ǣ�������| A��ԭ�Ӱ뾶��W��Z��Y��X | B������������Ӧˮ�������ԣ�Z��W��X | C��4��Ԫ�ص��⻯���У�Z�⻯�����ȶ� | D��W������ˮ��Ӧ������һ����Ư�������� |
 ��ͼ��Ԫ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ�X��Z��������֮��Ϊ21������˵����ȷ���ǣ�������
��ͼ��Ԫ�����ڱ���һ���֣�X��Y��Z��W��Ϊ������Ԫ�أ�X��Z��������֮��Ϊ21������˵����ȷ���ǣ�������| A��XԪ�������γ����������� | B��YԪ�ص�����������ˮ�����Ǻ�������������ǿ�� | C��XԪ�صķǽ����Ա�YԪ�طǽ�����ǿ | D��Z��X���Թ��ۼ�����γ�һ�����ǽ������� |
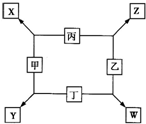 �ס��ҡ������������ɶ�����Ԫ����ɵĵ��ʣ�X��Y��Z��W��Ϊ����������£�XΪ����ɫ���壬Z��������Ϊ����ɫ��W��ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ҵ�ϳ���W����ȡZ����ת����ϵ����ͼ��ʾ��
�ס��ҡ������������ɶ�����Ԫ����ɵĵ��ʣ�X��Y��Z��W��Ϊ����������£�XΪ����ɫ���壬Z��������Ϊ����ɫ��W��ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ҵ�ϳ���W����ȡZ����ת����ϵ����ͼ��ʾ��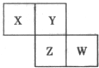 ��2012?��ɽ��ģ��X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵������ȷ���ǣ�������
��2012?��ɽ��ģ��X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵������ȷ���ǣ�������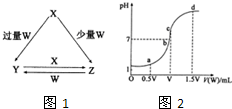 ��2013?�����ģ��X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ1��ʾ��ˮ�����ֲ�������ȥ����
��2013?�����ģ��X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ1��ʾ��ˮ�����ֲ�������ȥ����