题目内容
部分金属可以与水在一定条件下发生反应。
(1)将一小块金属钠投入盛有水的烧杯中,待完全反应后,向其中滴加酚酞溶液。①钠与水反应的化学方程式是 。
②上述实验过程中,可观察到的实验现象有 (填选项序号),
产生现象b的原因是 。
a.钠浮在水面 b.钠熔成小球
c.钠球在水面上四处游动,发出嘶嘶声,逐渐消失
d.反应后向烧杯中滴加酚酞溶液,溶液呈红色
(2)铁粉可以与水蒸气在高温下反应,生成 (填化学式)和氢气。
(1)①2Na + 2H2O == 2NaOH + H2↑ ②a b c d 反应放出大量的热,钠的熔点较低(2)Fe3O4
解析试题分析:(1)钠与水反应的化学方程式是①2Na + 2H2O == 2NaOH + H2↑。②由于Na的密度比水小,所以浮在水面上;由于Na的熔点低,Na与水的反应又是放热反应,反应放出的热是Na熔化,所以钠熔成小球;Na与水反应产生氢气,氢气泡破裂产生嘶嘶声,由于Na球受到的各个方向的力大小不等,所以在水面上四处游动; Na与水反应产生NaOH,使溶液显碱性,滴加酚酞试剂,溶液变为红色。(2)铁粉可以与水蒸气在高温下反应,方程式为3Fe+4H2O(g)  Fe3O4+4H2↑。可见反应产物有Fe3O4和氢气。
Fe3O4+4H2↑。可见反应产物有Fe3O4和氢气。
考点:考查金属Na、Fe与水反应的条件、现象及反应产物、方程式的书写的知识。

下列金属单质中,不能从溶液中置换出铜的是( )
| A.Zn | B.Al | C.Na | D.Fe |
2003年诺贝尔化学奖授予了美国科学家Peter Agre和Roderick Mackinnon以表彰他们在“水通道”和“离子通道”的研究成就。Mackinnon教授的研究内容主要是Na+、K+体积很接近,但在生物体内呈现的差别却高达1万倍,他革命性的让科学家观测Na+、K+在进入离子通道前、通道中以及穿过通道后的状态,可为病人在“离子通道”中寻找具体的病因,并研制相应药物。下列关于钠、钾的说法正确的是
| A.单质钠的密度比钾的密度小 |
| B.Na+和K+常用作氧化剂 |
| C.钠和钾都是短周期元素 |
| D.钠和钾的合金[ω(K)=50%~80%]在室温下呈液态 |
将Cu片放入0.1 mol/L FeCl3溶液中,反应一定时间后取出Cu片,溶液中C(Fe3+):C(Fe2+)=2:3,则Cu2+与Fe3+的物质的量之比为 ( )
| A.3:2 | B.3:5 | C.4:3 | D.3:4 |
工业上焙烧明矾[ KAl(SO4)2·12H2O]的化学方程式为:
4 KAl(SO4)2·12H2O+3S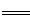 2K2SO4+2Al2O3 +9SO2 +48H2O,下列判断正确的是
2K2SO4+2Al2O3 +9SO2 +48H2O,下列判断正确的是
| A.在焙烧明矾的反应中,还原剂与氧化剂的物质的量之比是3:4 |
| B.最后得到的K2SO4溶液呈中性,所以c(K+)=c(SO42-) |
| C.焙烧产生的SO2可用于制硫酸,焙烧948 t明矾(M=" 474" g/mol),若SO2的利用率为96%,可生产质量分数为98%的硫酸432 t |
| D.工业上冶炼Al2O3制得Al,以Al和NiO(OH)为电极,NaOH溶液为电解液组成一种新型电池,放电时NiO(OH)转化为Ni(OH)2,该电池反应的化学方程式是 |
 NaAlO2+3Ni(OH)2
NaAlO2+3Ni(OH)2 取一小块钠,用滤纸去掉煤油,放在石棉网上加热,下列实验现象的描述正确的是 ①金属钠先熔化 ②在空气中燃烧呈苍白色火焰且火花四溅 ③燃烧后得到白色固体 ④燃烧时有黄色火焰 ⑤燃烧后生成淡黄色固体
| A.①②⑤ | B.①④⑤ | C.①②③ | D.①②④ |
下列各图与表述一致的是
| A.图①可以表示对某化学平衡体系改变温度后反应速率随时间的变化 |
| B.图②b曲线表示反应CH2=CH2(g)+H2(g)→CH3-CH3(g)ΔH <0,使用催化剂时,反应过程中的能量变化 |
| C.曲线图③可以表示向一定量的氢氧化钠溶液中滴加一定浓度氯化铝溶液时产生沉淀的物质的量变化 |
| D.图④电解饱和食盐水的装置中阴极的电极反应式为:2H++ 2e- = H2↑ |
下列各组物质相互反应后,再向得到的溶液中滴入KSCN试剂,溶液变成红色的是
| A.氯水和氯化亚铁溶液 | B.铁屑和氯化铜溶液 |
| C.铁屑和过量稀硫酸 | D.过量铁屑和氯化铁溶液 |
1 L某混合溶液中,溶质X、Y浓度都为0.1mol·L—1,向混合溶液中滴加0.1 mol·L—1某溶液Z,所得沉淀的物质的量如图所示,则X、Y、Z分别是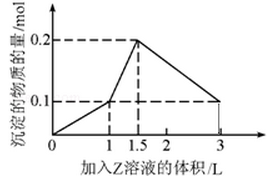
| A.氯化铝、氯化镁、氢氧化钠 |
| B.偏铝酸钠、氢氧化钡、硫酸 |
| C.氯化铝、氯化铁、氢氧化钠 |
| D.偏铝酸钠、氯化钡、硫酸 |