��Ŀ����
|
�����Ȼ�ѧ����ʽ��˵����ȷ���� | |
| [����] | |
A�� |
�����ȼ����Ϊ��H����890 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)��2O2(g) |
B�� |
500�桢30 MPa�£���0.5 mol��N2��1.5 mol��H2�����ܱյ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪ�� N2(g)��3H2(g) |
C�� |
��֪��H2(g)��F2(g)��2HF(g)����H����270 kJ/mol����1 mol������1 mol������Ӧ����2 molҺ̬������ų�������С��270 kJ |
D�� |
��C����ͬ�����£�2 mol��HF���������С��1 mol������1 mol�����������ܺ� |

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д���7�֣���ѧ���ļ�����ָ��̬ԭ�Ӽ��γ�1mol��ѧ��ʱ�ͷŵ��������磺H(g)��I(g)��H��I(g)��297KJ ��H��I���ļ���Ϊ297kJ/mol��Ҳ��������Ϊ�ƻ�1mol H��I����Ҫ����297KJ����������ѧ��Ӧ�ķ������Կ��ɾɻ�ѧ�����ƻ����»�ѧ�����γɡ��±���һЩ�������ݡ�����λ��kJ/mol��
|
| ���� |
| ���� |
| ���� |
| H��H | 436 | Cl��Cl | 243 | H��Cl | 432 |
| S��S | 255 | H��S | 339 | C��F | 427 |
| C��Cl | 330 | C��I | 218 | H��F | 565 |
| C��O | 347 | H��O | 464 | Si��Si | 176 |
| Si��O | 460 | O=O | 497 |
|
|
�Ķ�������Ϣ���ش��������⣺
��1�����ݱ��������ж�CCl4���ȶ��� ������ڡ���С�ڡ���CF4���ȶ��ԡ���Ԥ��C��Br���ļ��ܷ�Χ_________< C��Br���� <__________
��2��������Ϊ��H��O���ļ��ܴ���H��S���ļ��ܣ�����H2O���۷е����H2S���۷е㡣���Ƿ���ͬ���ֹ۵㣿�粻��ͬ����˵����Ľ��͡�
��3����֪H2O(l)��H2O(g) ��H��+44kJ/mol,��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��16�֣���úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ��ú������
��1����ˮ����ͨ�����ȵ�̼���ɲ���ˮú������ӦΪ��
C(s)��H2O(g)CO(g)��H2(g) ��H=��131.3kJ•mol-1��
�ٸ÷�Ӧ�ڳ����� �Է����У���ܡ��롰���ܡ�����
�ں��£����ݻ��ɱ���ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬����
����������ܶȣ� �������������ѹǿ��
��������������ʵ����� ��CO���ʵ���Ũ��
A��ֻ�Т� B��ֻ�Т�͢� C��ֻ�Т�͢� D����͢�
��2��ˮú���ٽ�һ����Ӧ����ȡ��������ӦΪH2O(g)+CO(g) H2(g)+CO2(g)��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K= 4/9�����¶����ڼס��ҡ������������ܱ������У�ֻͶ��H2(g)��CO2(g)������ʼŨ�����±���ʾ�������жϲ���ȷ���� ��
| ��ʼŨ�� | �� | �� | �� |
| c(H2)/mol/L | 0.010 | 0.020 | 0.020 |
| c(CO2)/mol/L | 0.010 | 0.010 | 0.020 |
A����Ӧ��ʼʱ�����еķ�Ӧ������죬���еķ�Ӧ��������
B��ƽ��ʱ�����кͱ��У�2��ת���ʾ���60��
C��ƽ��ʱ�����У�(CO2)�Ǽ��е�2������0.012mol/L
D��ƽ��ʱ������CO2��ת���ʴ���60%
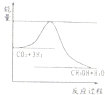
��3��Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2(g)��3H2(g)CH3OH(g)��H2O(g) ����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ•mol��1)�ı仯�������Ϊ1 L�ĺ����ܱ������У�����1mol CO2��3mol H2��Ӧ
�����д�ʩ����ʹc (CH3OH)������� ��
A�������¶�
B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з������
D���ٳ���1molCO2��3molH2
�����¶�T1ʱ������Ӧ�ﵽƽ��ʱ�����n(H2) =2.4 mol�������������䣬���¶�T2ʱ������Ӧ�ﵽƽ��ʱ�����n(CO2) =0.82 mol����T2 T1�������������������������
��4����һ�������¿�ѧ�Ҵ��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״���CH3OH��H2��ȼ���ȷֱ�Ϊ����H����725.5kJ/mol����H����285.8kJ/mol��
��д����ҵ����CO2��H2�ϳ�CH3OH��Һ̬ˮ���Ȼ�ѧ����ʽ�� ��
�ڸ�ת���Ļ��������� ��
�����������������Ʒ�ӦCO2��C��O2����H��0����S��0��������CO2�Ի�����Ӱ�졣�����ж��Ƿ���в�˵�����ɣ�
��7�֣���ѧ���ļ�����ָ��̬ԭ�Ӽ��γ�1mol��ѧ��ʱ�ͷŵ��������磺H(g)��I(g)��H��I(g)��297KJ ��H��I���ļ���Ϊ297kJ/mol��Ҳ��������Ϊ�ƻ�1mol H��I����Ҫ����297KJ����������ѧ��Ӧ�ķ������Կ��ɾɻ�ѧ�����ƻ����»�ѧ�����γɡ��±���һЩ�������ݡ�����λ��kJ/mol��
|
|
���� |
|
���� |
|
���� |
|
H��H |
436 |
Cl��Cl |
243 |
H��Cl |
432 |
|
S��S |
255 |
H��S |
339 |
C��F |
427 |
|
C��Cl |
330 |
C��I |
218 |
H��F |
565 |
|
C��O |
347 |
H��O |
464 |
Si��Si |
176 |
|
Si��O |
460 |
O=O |
497 |
|
|
�Ķ�������Ϣ���ش��������⣺
��1�����ݱ��������ж�CCl4���ȶ��� ������ڡ���С�ڡ���CF4���ȶ��ԡ���Ԥ��C��Br���ļ��ܷ�Χ_________< C��Br���� <__________
��2��������Ϊ��H��O���ļ��ܴ���H��S���ļ��ܣ�����H2O���۷е����H2S���۷е㡣���Ƿ���ͬ���ֹ۵㣿�粻��ͬ����˵����Ľ��͡�
��3����֪H2O(l)��H2O(g) ��H��+44kJ/mol,��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��16�֣���úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ��ú������
��1����ˮ����ͨ�����ȵ�̼���ɲ���ˮú������ӦΪ��
C(s)��H2O(g)
 CO(g)��H2(g) ��H=��131.3
kJ•mol-1��
CO(g)��H2(g) ��H=��131.3
kJ•mol-1��
�ٸ÷�Ӧ�ڳ����� �Է����У���ܡ��롰���ܡ�����
�ں��£����ݻ��ɱ���ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬����
����������ܶȣ� �������������ѹǿ��
��������������ʵ����� ��CO���ʵ���Ũ��
A��ֻ�Т� B��ֻ�Т�͢� C��ֻ�Т�͢� D����͢�
��2��ˮú���ٽ�һ����Ӧ����ȡ��������ӦΪH2O(g)+CO(g) H2(g)+CO2(g)��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K= 4/9�����¶����ڼס��ҡ������������ܱ������У�ֻͶ��H2(g)��CO2(g)������ʼŨ�����±���ʾ�������жϲ���ȷ���� ��
H2(g)+CO2(g)��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K= 4/9�����¶����ڼס��ҡ������������ܱ������У�ֻͶ��H2(g)��CO2(g)������ʼŨ�����±���ʾ�������жϲ���ȷ���� ��
|
��ʼŨ�� |
�� |
�� |
�� |
|
c(H2)/mol/L |
0.010 |
0.020 |
0.020 |
|
c(CO2)/mol/L |
0.010 |
0.010 |
0.020 |
A����Ӧ��ʼʱ�����еķ�Ӧ������죬���еķ�Ӧ��������
B��ƽ��ʱ�����кͱ��У�2��ת���ʾ���60��
C��ƽ��ʱ�����У�(CO2)�Ǽ��е�2������0.012mol/L
D��ƽ��ʱ������CO2��ת���ʴ���60%
��3��Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g) ����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ•mol��1)�ı仯�������Ϊ1 L�ĺ����ܱ������У�����1mol CO2��3mol H2��Ӧ
CH3OH(g)��H2O(g) ����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ•mol��1)�ı仯�������Ϊ1 L�ĺ����ܱ������У�����1mol CO2��3mol H2��Ӧ

�����д�ʩ����ʹc (CH3OH)������� ��
A�������¶�
B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з������
D���ٳ���1mol CO2��3mol H2
�����¶�T1ʱ������Ӧ�ﵽƽ��ʱ�����n(H2) = 2.4 mol�������������䣬���¶�T2ʱ������Ӧ�ﵽƽ��ʱ�����n(CO2) = 0.82 mol����T2 T1�������������������������
��4����һ�������¿�ѧ�Ҵ��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״���CH3OH��H2��ȼ���ȷֱ�Ϊ����H����725.5kJ/mol����H����285.8kJ/mol��
��д����ҵ����CO2��H2�ϳ�CH3OH��Һ̬ˮ���Ȼ�ѧ����ʽ�� ��
�ڸ�ת���Ļ��������� ��
�����������������Ʒ�ӦCO2��C��O2����H��0����S��0��������CO2�Ի�����Ӱ�졣�����ж��Ƿ���в�˵�����ɣ�