��Ŀ����
(7��)һ���¶��£���3molA�����1 molB����ͨ��һ�ܱ������У��������·�Ӧ��
![]() ������д���пհף�
������д���пհף�
(1)����������̶�Ϊ2L����Ӧ2minʱ���ʣ��O. 6molB��C��Ũ��Ϊ0. 4 mol/L����
�� x=______________��
������Ӧ��4min�ﵽƽ�⣮ƽ��ʱC��Ũ��___________O.8mol��L(����ڡ��������ڡ�����С�ڡ�)
��ƽ�������У�C���������Ϊ22������B��ת������______________��
�ܸı���ʼ������������ʹ��Ӧ�ﵽƽ��ʱ��C�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ���n(A)��n(B)��n(c)֮��Ӧ������Ĺ�ϵʽΪ_____________________________________________��
(2)��ά������ѹǿ���䣬
�ٴﵽƽ��ʱC���������____________22����(����ڡ��������ڡ���С�ڡ�)
�ڸı���ʼ���ʼ���������ʹ��Ӧ�ﵽƽ��ʱ��C�����ʵ�����ԭƽ���2������A��B����ʼ���ʵ����ֱ�ӦΪ__________________________��
��1���� ![]() ��1�֣�
��1�֣�
�� С�� ��1�֣�
��B��ת���ʣ�36% ��1�֣�
��![]() ����Ĺ�ϵʽΪ��
����Ĺ�ϵʽΪ��
![]() ��2�֣�
��2�֣�
��2���� ���� ��1�֣�
�� ![]() ��1�֣�
��1�֣�

 xC(g)������д���пհף�
xC(g)������д���пհף�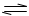 bM��g����M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
bM��g����M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��