��Ŀ����
��1��д��������ˮ��Һ�еĵ��뷽��ʽ ����ij�¶��£�CH3COOH(aq)��NaOH(aq)��Ӧ�ġ�H=" -" 46.8kJ��mol-1��HCl(aq)��NaOH(aq)��Ӧ�ġ�H=" -" 55.6 kJ��mol-1����CH3COOH��ˮ��Һ�е���ġ�H= kJ��mol-1��
��2��ij�¶��£�ʵ����0.1mol��L-1��������ԼΪ1.5%������¶���0.1mol��L-1CH3COOH�ĵ���ƽ�ⳣ��K=________���г�����ʽ����֪����� ��
��
��3����������ѧ���о�����������ϩ������Ϊԭ�ϡ��Ӷ����������ϳ������������¹��գ����������Ҵ�����ȩ���м��壬ʹ��Ʒ�ɱ����ͣ��������Ծ������ơ���ϳɵĻ�����Ӧ���£�

����������˵����ϩ������ϳ����������ķ�Ӧ�Ѵﻯѧƽ����� ��
A����ϩ�����ᡢ����������Ũ����ͬ |
B�������ϳɷ�Ӧ�����������ֽⷴӦ��������� |
C����ϩ�Ͽ�1mol̼̼˫����ͬʱ����ǡ������1mol |
D����ϵ����ϩ�İٷֺ���һ�� |
��4����n(��ϩ)��n(����)���ϱ�Ϊ1�������£�ij�о�С���ڲ�ͬѹǿ�½���������ͬʱ������������IJ������¶ȵı仯�IJⶨʵ�飬ʵ������ͼ��ʾ���ش��������⣺

�� �¶���60��80�淶Χ�ڣ���ϩ�����������ϳɷ�Ӧ�����ɴ�С��˳���� [�� (P1)��
(P1)�� (P2)��
(P2)�� (P3)�ֱ��ʾ��ͬѹǿ�µķ�Ӧ����]��������ԭ��Ϊ ��
(P3)�ֱ��ʾ��ͬѹǿ�µķ�Ӧ����]��������ԭ��Ϊ ��
��ѹǿΪP1MPa���¶�60��ʱ�������������IJ���Ϊ30�G�����ʱ��ϩ��ת����Ϊ ��
����ѹǿΪP1MPa���¶ȳ���80��ʱ���������������½���ԭ�������_________��
�ܸ��ݲⶨʵ���������������˵����������� ��������ʵ�ѹǿ���¶ȣ���Ϊ������������ĺϳ����ʺͲ��ʣ����Բ�ȡ�Ĵ�ʩ�� ����д��һ������
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д���ҵ�����Ȼ�隣������̿��Ʊ�̼���̵���������ͼ��ʾ��
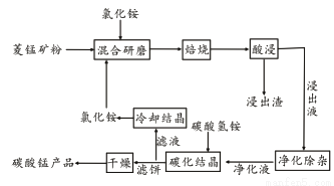
��֪�������̿����Ҫ�ɷ���MnCO3 �����к�Fe��Ca��Mg��Al��Ԫ�ء�
�ڱ��չ�������Ҫ��ӦΪ��MnCO3 +2NH4Cl  MnCl2+2NH3�� +CO2��+ H2O��
MnCl2+2NH3�� +CO2��+ H2O��
�۲��������ӳ���ʱ��Һ��pH��ֵ��
Al3- | Fe3+ | Ca2+ | Mn2+ | Mg2+ | |
��ʼ������pHֵ | 4.1 | 2.2 | 10.6 | 8.8 | 9.6 |
������ȫ��pHֵ | 4.7 | 3.2 | 13.1 | 10.1 | 11.1 |
��1��ʵ���ҡ����ա�����ʢ�Ź��������Ϊ____________________��
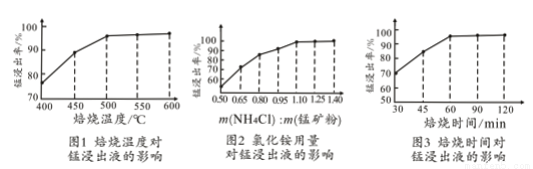
��2�����ͼ1��2��3���������չ����ж��¶ȡ�NH4Cl����[m��NH4Cl����m���̿�ۣ�]��ʱ������ѡ������Ϊ________________��_______________��______________��
��3�����̽���Һ��������ʱ���ȼ���MnO2��Fe2��ת��ΪFe3�����ٵ�����ҺpH�ķ�ΧΪ_________________����Fe3����Al3����Ϊ������ȥ��Ȼ�����NH4F��Ca2����Mg2����Ϊ�����������ȥ��
��4����̼���ᾧ�������У�����̼������Ƿ�Ӧ�����ӷ���ʽΪ________________��
��5�����������п�ѭ��ʹ�õ�������_______________��
��6��Ϊ�ⶨ��Ʒ��̼���̵ĺ������������ʵ�飨���ʲ��μӷ�Ӧ����ʵ�鲽��Ϊ����ȡ16.80g����������������ϡ������Һ�У���������Һ�м����Թ�������������ᣬ����ʹ��Ӧ��2Mn2++NO3-+4PO43-+2H+ 2[Mn(PO4)2]3-+NO2-+H2O��ֽ��С���ȥ��Һ�д��ڵ�NO3-��NO2-����l00.00mL2.00 mol��L-1��(NH4)2Fe(SO4)2��Һ�������ķ�ӦΪ��[Mn(PO4)2]3-+Fe2��=Mn2++Fe3��+2PO3-������1.00mol��L-1����K2Cr2O7��Һ�ζ�������Fe2�����ζ��յ�ʱ����10.00mL����K2Cr2O7��Һ��
2[Mn(PO4)2]3-+NO2-+H2O��ֽ��С���ȥ��Һ�д��ڵ�NO3-��NO2-����l00.00mL2.00 mol��L-1��(NH4)2Fe(SO4)2��Һ�������ķ�ӦΪ��[Mn(PO4)2]3-+Fe2��=Mn2++Fe3��+2PO3-������1.00mol��L-1����K2Cr2O7��Һ�ζ�������Fe2�����ζ��յ�ʱ����10.00mL����K2Cr2O7��Һ��
������K2Cr2O7��Һ��Fe2����Ӧ�����ӷ���ʽΪ_____________________����ԭ������Cr3������
�ڲ�Ʒ��̼���̵���������Ϊ_____________���������3λ��Ч���֣���


 B.
B.  C.
C.  D.
D. 

