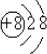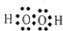��Ŀ����
��ԭ��������С��20��A��B��C��D����Ԫ�أ���֪��
�� A��B��ͬһ���壬C��D��ͬһ���ڣ�
�� ��Ԫ�������γ�A2C��A2C2��B2C2��DC2�Ȼ����
�� B����������C�������Ӻ�����Ӳ�ṹ��ͬ��
��B2C2��A2C��DC2��Ӧ������C2���壻
�� B�ĵ�����A2C��Ӧ����A2���壬A2��C2�������ɷ�����ը�����ɳ�������ɫ����ζ��Һ��A2C��
�Իش�
��1��д��A��D��Ԫ�ص����ƣ�A________��D________
��2������B���Ӻ�C���ӵĽṹʾ��ͼ_____________ �� _____________ �����������У��뾶��С����
________�������ӷ��ţ���
��3��д��A2C2��B2C2�ĵ���ʽ��A2C2__________B2C2__________ ��
��4��д��B2C2��DC2��Ӧ�Ļ�ѧ����ʽ______________________________
�� A��B��ͬһ���壬C��D��ͬһ���ڣ�
�� ��Ԫ�������γ�A2C��A2C2��B2C2��DC2�Ȼ����
�� B����������C�������Ӻ�����Ӳ�ṹ��ͬ��
��B2C2��A2C��DC2��Ӧ������C2���壻
�� B�ĵ�����A2C��Ӧ����A2���壬A2��C2�������ɷ�����ը�����ɳ�������ɫ����ζ��Һ��A2C��
�Իش�
��1��д��A��D��Ԫ�ص����ƣ�A________��D________
��2������B���Ӻ�C���ӵĽṹʾ��ͼ_____________ �� _____________ �����������У��뾶��С����
________�������ӷ��ţ���
��3��д��A2C2��B2C2�ĵ���ʽ��A2C2__________B2C2__________ ��
��4��д��B2C2��DC2��Ӧ�Ļ�ѧ����ʽ______________________________
��1���⣻̼
��2�� ��
��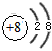 ��Na+
��Na+
��3��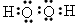 ��
��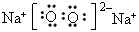
��4��2Na2O2+2CO2==2Na2CO3+O2
��2��
 ��
��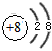 ��Na+
��Na+ ��3��
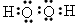 ��
��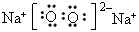
��4��2Na2O2+2CO2==2Na2CO3+O2

��ϰ��ϵ�д�
�����Ŀ
| |||||||||||||||