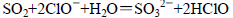��Ŀ����
���л�ѧ��Ӧ�����ӷ���ʽ����ȷ����
A��Na2S��Һ�м�������FeCl3��Һ��2Fe3+��S2�� 2Fe2+��S�� 2Fe2+��S�� |
| B����NaOH��Һ�еμ�̼�������Һ��OH��ǡ����ȫ��Ӧ�� Ca2++2OH����2HCO3��  CaCO3����2H2O��CO32�� CaCO3����2H2O��CO32�� |
C����NaClO��Һ��ͨ������SO2���壺ClO����SO2��H2O SO42����Cl����2H+ SO42����Cl����2H+ |
D��Na2CO3��Һ�е����������ǻ���������Һ�� ��CO32�� ��CO32��  ��HCO3�� ��HCO3�� |
AD
���������A��Na2S��Һ�м�������FeCl3��Һ��������FeS��S��A����ȷ��B����NaOH��Һ�еμ�̼�������Һ��OH��ǡ����ȫ��Ӧʱ��������̼��ơ�̼���ƺ�ˮ��B��ȷ��C����NaClO��Һ��ͨ������SO2��������ӷ���ʽ��ClO����SO2��H2O
 SO42����Cl����2H+��C��ȷ��D�����ǻ�������ǿ��HCO3���ģ�����Na2CO3��Һ�е����������ǻ���������Һʱ���ǻ�Ҳ��̼���Ʒ�Ӧ��D����ȷ����ѡAD��
SO42����Cl����2H+��C��ȷ��D�����ǻ�������ǿ��HCO3���ģ�����Na2CO3��Һ�е����������ǻ���������Һʱ���ǻ�Ҳ��̼���Ʒ�Ӧ��D����ȷ����ѡAD��
��ϰ��ϵ�д�
�����Ŀ
 H3O++S2-
H3O++S2- Cl2����H2��
Cl2����H2�� 2Fe(OH)3(s)��3Mg2+
2Fe(OH)3(s)��3Mg2+ + CO2 +H2O��
+ CO2 +H2O��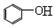 + HCO3-
+ HCO3-