��Ŀ����
����Ŀ�������£�N2H4���ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�á��ش��������⣺
��1����֪��N2(g)+3H2(g) ![]() 2NH3(g) ��H��-92.4kJ��mol-1
2NH3(g) ��H��-92.4kJ��mol-1
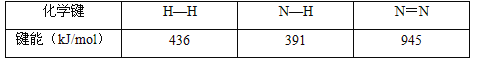
�ں��¡����ݵ��ܱ������У��ϳɰ���Ӧ�ĸ�����Ũ�ȵı仯������ͼ��ʾ��
�� �����ڸ��¶��·�Ӧ2NH3(g) ![]() N2(g)+3H2(g)��ƽ�ⳣ��K=________��
N2(g)+3H2(g)��ƽ�ⳣ��K=________��
�� �ڵ�25minĩ�����������������䣬�����¶Ƚ��ͣ��ڵ�35minĩ�ٴδﵽƽ�⡣��ƽ���ƶ�������N2Ũ�ȱ仯��0.5mol/L������ͼ�л���25-40minNH3Ũ�ȱ仯���ߡ�________
�� ��֪��2N2(g)+6H2O(l) ![]() 4NH3(g)+3O2��g����H=+1530.0KJ/mol����������ֵΪ_____��
4NH3(g)+3O2��g����H=+1530.0KJ/mol����������ֵΪ_____��
��2���� N2H4��һ�ָ���ȼ�Ͼ��л�ԭ�ԣ�ͨ����NaClO�����NH3��Ӧ�Ƶã������Ϊʲô�ù���������Ӧ��ԭ��__________
�� ��NaClO��NH3 ��N2H4�ķ�Ӧ���൱���ӵģ���Ҫ��Ϊ������
��֪��һ����NH3+ClO-=OH-+NH2Cl
��д���ڶ������ӷ���ʽ��__________________
�� N2H4������ˮ�����백�����Ƶ������֪�䳣���µ��볣��K1=1.0��10-6�������£���0.2 mol/L N2H4��H2O��0.lmol/L������������ϣ���������仯�������ʱ��Һ��PH����________������N2H4�Ķ������룩��
���𰸡� 6.75  142.9 kJ ��g��1 ������NaClO���ܽ�N2H4����ΪN2���������ɣ� NH3+NH2Cl+OH����N2H4+Cl��+H2O 8
142.9 kJ ��g��1 ������NaClO���ܽ�N2H4����ΪN2���������ɣ� NH3+NH2Cl+OH����N2H4+Cl��+H2O 8
��������(l)�� ͼ��A��C�ı仯Ũ��֮��Ϊ3:1����AΪH2��CΪN2��BΪNH3��ƽ��ʱc(H2)Ϊ3.0mol/L��c(N2)Ϊ1.0mol/L��c(NH3)Ϊ2.0mol/L���ڸ��¶��·�Ӧ2NH3(g) ![]() N2(g)+3H2(g)��ƽ�ⳣ��K=
N2(g)+3H2(g)��ƽ�ⳣ��K=![]() =6.75��
=6.75��
���ڵ�25minĩ�����������������䣬��ӦN2(g)+3H2(g) ![]() 2NH3(g) ��H��-92.4kJ��mol-1��������ȣ������¶Ƚ��ͣ�ƽ�������ƶ�������Ũ������NH3Ũ�ȱ仯����Ϊ
2NH3(g) ��H��-92.4kJ��mol-1��������ȣ������¶Ƚ��ͣ�ƽ�������ƶ�������Ũ������NH3Ũ�ȱ仯����Ϊ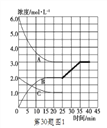 ��
��
�� ��֪����2N2(g)+6H2O(l) ![]() 4NH3(g)+3O2(g)��H=+1530.0KJ/mol����N2(g)+3H2(g)
4NH3(g)+3O2(g)��H=+1530.0KJ/mol����N2(g)+3H2(g) ![]() 2NH3(g) ��H��-92.4kJ��mol-1��[����2-��]��6�ɵ�H2(g) +
2NH3(g) ��H��-92.4kJ��mol-1��[����2-��]��6�ɵ�H2(g) +![]() O2(g)=H2O(l) ����H��[(-92.4kJ��mol-1)��2-(+1530.0KJ/mol)]��6=-285.8kJ ��mol��1����2g������ȫȼ�շų�������Ϊ285.8kJ ������������ֵΪ142.9 kJ ��g��1��
O2(g)=H2O(l) ����H��[(-92.4kJ��mol-1)��2-(+1530.0KJ/mol)]��6=-285.8kJ ��mol��1����2g������ȫȼ�շų�������Ϊ285.8kJ ������������ֵΪ142.9 kJ ��g��1��
(2)�� NaClO��ǿ�����ԣ����NaClO�����ܽ�N2H4����ΪN2����ͨ����NaClO�����NH3��Ӧ��N2H4����N2H4��NaClO������
��NaClO��NH3��N2H4���ܷ�Ӧ��NaClO+2NH3=N2H4+NaCl+H2O�������ӷ�Ӧ����ʽΪClO-+2NH3=N2H4+Cl-+H2O����ڶ������ӷ���ʽ�����ܷ�Ӧ���ӷ��̼�ȥ��һ����NH3+ClO-=OH-+NH2Cl ��NH3+NH2Cl+OH����N2H4+Cl��+H2O ��
������0.2mo1L-1N2H4H2O��Һ��0.1molL-1HCl��Һ�������ϣ��õ����ʵ�Ũ�����N2H5C1��N2H4H2O��N2H5++H2O![]() N2H4H2O+H+��c(H+)=
N2H4H2O+H+��c(H+)=![]() =
=![]() mol/L=1��10-8mol/L��pH=8��
mol/L=1��10-8mol/L��pH=8��

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�