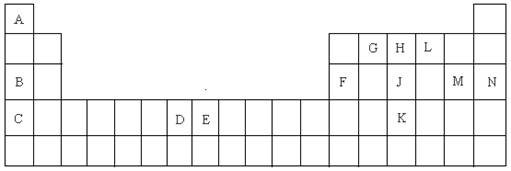
��Ŀ����
��15�֣�������Ԫ�����ڱ���һ���֣����ݸ�����Ԫ�ػش���������
��1��K��Ԫ�ط����� ��
��2��DԪ�ص���Χ�����Ų�ͼ�� �����仯�����У���������� ��
��3��CԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4��A��L��B��L���ֱ����γ�ԭ�Ӹ�����Ϊ1��1�Ļ����A��L��1��1���Ļ������� ���ӣ���Ի�Ǽ��ԣ���B��L��1��1���Ļ�����ĵ���ʽ�� ��
��5��G��H��Lԭ�ӵĵ�һ�������ɸߵ��͵�˳���� ����Ԫ�ط��ţ���
��6��J���ʵ�һ�ְ�ɫͬ���칹��Ŀռ乹���� ��
��7��EԪ����Ԫ�����ڱ��е�λ���� �����仯�����У����У�2����3�۵�2�����ӣ���3�����ӱȽ��ȶ�����ԭ���� ��
��8��A��B�Ļ�������۵��A��L�Ļ�������۵�ߣ���ԭ���� ��
��9��A�ĵ�����L�ĵ�����B������������ˮ������Һ�����ȼ�յ�أ�д�����ֵ�صĸ����ĵ缫��Ӧʽ ��
��10��FԪ�ص������������MԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ��
��
��1�� As ����2���� ��7 ����3��
��
��4�� ���ԣ� ����5�� N O C ����6�� �������� ��
��7�� �������ڵڢ��� �� Fe3����3d���Ϊ������ṹ�����ȶ� ��
��8�� NaH�����ӻ����H2O�ǹ��ۻ����� ����9��H2��2e����2OH�D��2H2O ��
��10�� Al2O3��6HClO4��2Al(ClO4)3��3H2O ��9��10ÿ��2�֣�����ÿ��1�֣�
����:

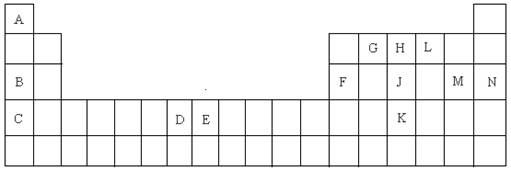

 ������
������ Ϊֱ���εķǼ��Է��ӡ�
Ϊֱ���εķǼ��Է��ӡ�
 �ĵ��ʻ�ѧ����ʽΪ___________________________��
�ĵ��ʻ�ѧ����ʽΪ___________________________�� ��Һ����
��Һ���� ���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ��__________________��
���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ��__________________��