��Ŀ����
ij��γ�С������50 mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ�������CO2��������NaHCO3�����������ʵ�鲽�裺
a��ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b��С�������Һ1��2 min�������ܽ�����Һ�е�CO2���壻
c���ڵõ�����Һ�м�����һ��(25mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
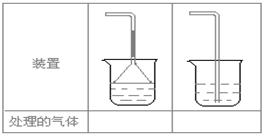
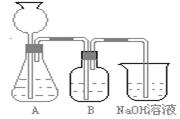
��1���˷������Ƶýϴ�����Na2CO3��д��c��������ӷ���ʽ_________________���˷�����һ����ʵ��װ������ͼ��ʾ
a��ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b��С�������Һ1��2 min�������ܽ�����Һ�е�CO2���壻
c���ڵõ�����Һ�м�����һ��(25mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
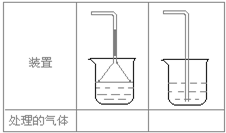
��1���˷������Ƶýϴ�����Na2CO3��д��c��������ӷ���ʽ_________________���˷�����һ����ʵ��װ������ͼ��ʾ

��2�����뷴Ӧ��ǰ����μ�������װ�õ������ԣ� __________________________________________
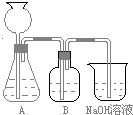
��3�����ô���ʯ��������CO2����װ��B��ʢ�ŵ��Լ���______________��������___________________________________
��4����ʵ����ͨ���Ʒ��У�װ��A������Ϊ����_________ (�����)����ķ���װ��
��HCl����H2����Cl2����NH3
��5����֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1��44 g/mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ_______ mol/L��
��3�����ô���ʯ��������CO2����װ��B��ʢ�ŵ��Լ���______________��������___________________________________
��4����ʵ����ͨ���Ʒ��У�װ��A������Ϊ����_________ (�����)����ķ���װ��
��HCl����H2����Cl2����NH3
��5����֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1��44 g/mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ_______ mol/L��
��1��HCO3-+OH-==CO32-+H2O
��2����һ�μ��A��B�������ԣ���ֹˮ�м�סB��C����齺�ܣ�Ȼ���©����ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©������ֱ���A��B�������ԣ��õ��ɼм�סA��B����齺�ܣ��ȼ��A�������ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿڽ���ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����
��3������̼��������Һ������HCl����
��4���ڡ�
��5��7.2 mol/L
��2����һ�μ��A��B�������ԣ���ֹˮ�м�סB��C����齺�ܣ�Ȼ���©����ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©������ֱ���A��B�������ԣ��õ��ɼм�סA��B����齺�ܣ��ȼ��A�������ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿڽ���ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����
��3������̼��������Һ������HCl����
��4���ڡ�
��5��7.2 mol/L

��ϰ��ϵ�д�
�����Ŀ
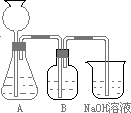
 (1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��
(1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��