��Ŀ����
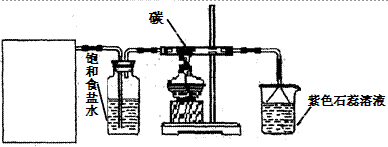
��16�֣�ijʵ��С����Ũ�����MnO2��ȡ��̽��Cl2��ѧ���ʣ����װ��ͼ����ͼ��ʾ��
��1��A����������װ�ã�ʡ��δ�������Ʊ�Cl2�Ļ�ѧ��Ӧ���ӷ���ʽ�� �� Aװ�ó��˾ƾ��ơ�Բ����ƿ��õ��IJ��������� ��
��2����Cװ�ó����������к���CO2��HCl������Cװ���з����Ļ�ѧ��Ӧ����ʽΪ��
��
��3��ͬѧ����ʵ���з��֣�����ϡ�������Ũ������MnO2��ϼ���û���������ɣ����ǿ�ʼ̽���������²�������������ԭ��
�������������
����1��H����Ũ�Ȳ�����
����2�� ��
����3��_________________________ _ ��
�����ʵ�鷽��������ʵ�顣�ڴ������д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ�������ˮ��ŨH2SO4��NaCl���塢MnO2���塢1mol/Lϡ���ᡢ1mol/LAgNO3��Һ�����ۣ�KI��Һ
|
ʵ�鲽�� |
Ԥ����������� |
|
����1��ȡ����1mol/Lϡ�������Թ�A�У���������MnO2�� �� |
|
|
����2��ȡ����1mol/Lϡ�������Թ�B�У���������MnO2�� �� |
|
��1��MnO2��4H+��2Cl- Mn2+��Cl2����2H2O��2�֣� ��Һ©����2�֣�
Mn2+��Cl2����2H2O��2�֣� ��Һ©����2�֣�
��2��2Cl2+2H2O+C CO2+4HCl��2�֣�
CO2+4HCl��2�֣�
��3���ټ���2��Cl����Ũ�Ȳ����� ��1�֣�����3��Cl����H����Ũ�Ⱦ������� ��1�֣�
�ڣ�8�֣�
|
ʵ�鲽�� |
Ԥ����������� |
|
�μӼ���Ũ���ᣬ��������������ܵĽ��������ܲ�����ۣ�KI��Һ�У���������2�֣� |
�������ۣ�KI��Һ�����������1������ �������ۣ�KI��Һ�������������2�����3��������2�֣� |
|
��������NaCl��������������������ܵĽ��������ܲ�����ۣ�KI��Һ�У���������2�֣� |
�������ۣ�KI��Һ�����������2�������������ۣ�KI��Һ����������ϲ���1�Ľ��ۢڣ������3��������2�֣� |
�����������⿼������������ȡԭ����MnO2��4H+��2Cl- Mn2+��Cl2����2H2O
Mn2+��Cl2����2H2O
��1����ȡ�����ǹ�Һ���ȵķ�ʽ��һ���÷�Һ©��װҺ�壬��ƿ��Ϊ��Ӧ������
��2����������ȡ������ֻ��ȥ��HCl����û�и���ڼ�����������C��Ӧ������ΪCO2��HCl����֪��Ӧ��Ϊ������C��ˮ
��3��̽����������ȡԭ�����ı��������Ũ�ȣ��Ϳ��Դ�Cl����Ũ�ȡ�H����Ũ�ȶԷ�Ӧ��Ӱ����м��衢ʵ�顢��֤��

��16�֣�ijʵ��С����Ũ�����MnO2��ȡ��̽��Cl2��ѧ���ʣ����װ��ͼ����ͼ��ʾ��
A B C D
��1��A����������װ�ã�ʡ��δ�������Ʊ�Cl2�Ļ�ѧ��Ӧ���ӷ���ʽ�� ��Aװ�ó��˾ƾ��ơ�Բ����ƿ��õ��IJ��������� ��
��2����Cװ�ó����������к���CO2��HCl������Cװ���з����Ļ�ѧ��Ӧ����ʽΪ
��
��3��ͬѧ����ʵ���з��֣�����ϡ�������Ũ������MnO2��ϼ���û���������ɣ����ǿ�ʼ̽
���������²�������������ԭ��
�������������
����1��H����Ũ�Ȳ�����
����2�� ��
����3��__________________________ ��
�����ʵ�鷽��������ʵ�顣�ڴ������д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ�������ˮ��ŨH2SO4��NaCl���塢MnO2���塢1mol/Lϡ���ᡢ1mol/LAgNO3��Һ�����ۣ�KI��Һ
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����1mol/Lϡ�������Թ�A�У���������MnO2��
�� |
|
| ����2��ȡ����1mol/Lϡ�������Թ�B�У���������MnO2��
�� |
|
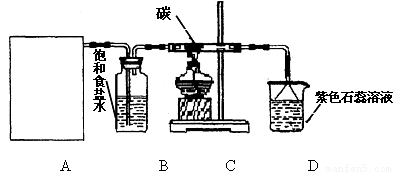
��16�֣�ijʵ��С����Ũ�����MnO2��ȡ��̽��Cl2��ѧ���ʣ����װ��ͼ����ͼ��ʾ��
��1��A����������װ�ã�ʡ��δ�������Ʊ�Cl2�Ļ�ѧ��Ӧ���ӷ���ʽ�� �� Aװ�ó��˾ƾ��ơ�Բ����ƿ��õ��IJ��������� ��
��2����Cװ�ó����������к���CO2��HCl������Cװ���з����Ļ�ѧ��Ӧ����ʽΪ��
��
��3��ͬѧ����ʵ���з��֣�����ϡ�������Ũ������MnO2��ϼ���û���������ɣ����ǿ�ʼ̽���������²�������������ԭ��
�������������
����1��H����Ũ�Ȳ�����
����2�� ��
����3��_________________________ _ ��
�����ʵ�鷽��������ʵ�顣�ڴ������д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ�������ˮ��ŨH2SO4��NaCl���塢MnO2���塢1mol/Lϡ���ᡢ1mol/LAgNO3��Һ�����ۣ�KI��Һ
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����1mol/Lϡ�������Թ�A�У���������MnO2�� �� | |
| ����2��ȡ����1mol/Lϡ�������Թ�B�У���������MnO2�� �� | |
��16�֣�ijʵ��С����Ũ�����MnO2��ȡ��̽��Cl2��ѧ���ʣ����װ��ͼ����ͼ��ʾ��

A B C D
��1��A����������װ�ã�ʡ��δ�������Ʊ�Cl2�Ļ�ѧ��Ӧ���ӷ���ʽ�� ��Aװ�ó��˾ƾ��ơ�Բ����ƿ��õ��IJ��������� ��
��2����Cװ�ó����������к���CO2��HCl������Cװ���з����Ļ�ѧ��Ӧ����ʽΪ
��
��3��ͬѧ����ʵ���з��֣�����ϡ�������Ũ������MnO2��ϼ���û���������ɣ����ǿ�ʼ̽
���������²�������������ԭ��
�������������
����1��H����Ũ�Ȳ�����
����2�� ��
����3��_________________________ _ ��
�����ʵ�鷽��������ʵ�顣�ڴ������д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ�������ˮ��ŨH2SO4��NaCl���塢MnO2���塢1mol/Lϡ���ᡢ1mol/LAgNO3��Һ�����ۣ�KI��Һ
|
ʵ�鲽�� |
Ԥ����������� |
|
����1��ȡ����1mol/Lϡ�������Թ�A�У���������MnO2��
�� |
|
|
����2��ȡ����1mol/Lϡ�������Թ�B�У���������MnO2��
�� |
|