��Ŀ����
��12�֣���A��B��C��D����Ԫ�أ���ԭ��������С��ϵΪA��B��C��D����֪����0.5 mol AԪ�ص�������ӻ�ԭ������ԭ��ʱ����õ�6.02��1023�����ӡ���A�ĵ� ��ͬ�����ַ�Ӧʱ�ɷų�0.02 g��������ȥ0.4 g A���ʡ�BԪ��ԭ�ӵĺ�����Ӳ�����A��ͬ����֪BԪ�ص�ԭ�Ӱ뾶��A��CԪ���γɵ��������������ǿ�ᣬҲ������ǿ�DԪ�����������ɵĻ�����Ļ�ѧʽΪDH3�����������������Ԫ�ص���������Ϊ74.07%���Իش�
��ͬ�����ַ�Ӧʱ�ɷų�0.02 g��������ȥ0.4 g A���ʡ�BԪ��ԭ�ӵĺ�����Ӳ�����A��ͬ����֪BԪ�ص�ԭ�Ӱ뾶��A��CԪ���γɵ��������������ǿ�ᣬҲ������ǿ�DԪ�����������ɵĻ�����Ļ�ѧʽΪDH3�����������������Ԫ�ص���������Ϊ74.07%���Իش�
��1��Ԫ�ط��ţ�A___��__��B___��__��C__��___��D__��__��
��2������ij�����к���BԪ�ص����ʵ����__��__��
��3��A��B��C����Ԫ������������Ӧˮ����ļ�����ǿ������˳���ǣ��û�ѧʽ��ʾ��__��____��
 ��ͬ�����ַ�Ӧʱ�ɷų�0.02 g��������ȥ0.4 g A���ʡ�BԪ��ԭ�ӵĺ�����Ӳ�����A��ͬ����֪BԪ�ص�ԭ�Ӱ뾶��A��CԪ���γɵ��������������ǿ�ᣬҲ������ǿ�DԪ�����������ɵĻ�����Ļ�ѧʽΪDH3�����������������Ԫ�ص���������Ϊ74.07%���Իش�
��ͬ�����ַ�Ӧʱ�ɷų�0.02 g��������ȥ0.4 g A���ʡ�BԪ��ԭ�ӵĺ�����Ӳ�����A��ͬ����֪BԪ�ص�ԭ�Ӱ뾶��A��CԪ���γɵ��������������ǿ�ᣬҲ������ǿ�DԪ�����������ɵĻ�����Ļ�ѧʽΪDH3�����������������Ԫ�ص���������Ϊ74.07%���Իش���1��Ԫ�ط��ţ�A___��__��B___��__��C__��___��D__��__��
��2������ij�����к���BԪ�ص����ʵ����__��__��
��3��A��B��C����Ԫ������������Ӧˮ����ļ�����ǿ������˳���ǣ��û�ѧʽ��ʾ��__��____��
��12�� ��2��
�� 1��Ca K Al
1��Ca K Al  N ��2����ɫ��Ӧ
N ��2����ɫ��Ӧ
��3��KOH��Ca��OH��2��Al��OH��3
��
 1��Ca K Al
1��Ca K Al  N ��2����ɫ��Ӧ
N ��2����ɫ��Ӧ��3��KOH��Ca��OH��2��Al��OH��3
��

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д�
�����Ŀ
 �õ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��
�õ�H��Һ�������������������ͬ����Ԫ���γɵļ��������л�ԭ����ǿ����������Ԫ�أ��������ʼ�ת����ϵ����ͼ�������ַ�Ӧ�����������ȥ��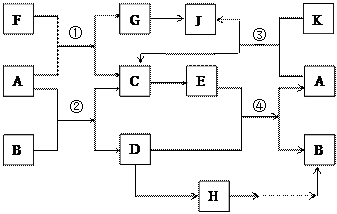

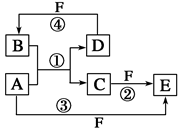
 ��Ԫ������������ˮ�����������ǿ���� ���ѧʽ�������л�ѧ�������Բ�ͬ���������ֻ������
��Ԫ������������ˮ�����������ǿ���� ���ѧʽ�������л�ѧ�������Բ�ͬ���������ֻ������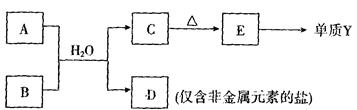
 �Խ�С�ڷ���
�Խ�С�ڷ��� �� �ֲ�ͬ�ܼ��ĵ��ӡ�
�� �ֲ�ͬ�ܼ��ĵ��ӡ�