��Ŀ����
11���������ӷ���ʽ��ȷ���ǣ�������| A�� | �Ȼ�����Һ�м��������ˮ��A13++4NH3•H2O�T4NH4++2H2O+A1O2- | |
| B�� | FeBr2��Һ��ͨ��������������2Fe2++2Br-+2C12�T2Fe3++Br2+4C1- | |
| C�� | ����������Һ�м����������ữ��˫��ˮFe2++2H++H2O2�TFe3++2H2O | |
| D�� | ��ͨ�����SO2���NaOH��Һ�м���������ˮ��������SO2���ܽ⣩��HSO3-+Br2+H2O�T3H++2Br-+SO42- |
���� A����ˮΪ������߷�Ӧ������������������
B�������������������Ӻ���������ȫ�����������������������ӵ����ʵ���֮��Ϊ2��1��
C�����ӷ���ʽ��������ɲ���ȣ�Υ���˵���غ㣻
D�������������ʱ�������������ƣ�������������ӱ��嵥����������������ӣ�
��� �⣺A���Ȼ����백ˮ��Ӧ�������������������Ȼ�泥���ȷ�����ӷ���ʽΪ��Al3++3NH3•H2O=Al��OH��3��+3NH4+����A����
B��FeBr2��Һ��ͨ����������������Ӧ�����嵥�ʺ��廯������ȷ�����ӷ���ʽΪ��2Fe2++4Br-+3C12�T2Fe3++2Br2+6C1-����B����
C������������Һ�м����������ữ��˫��ˮ�����߷���������ԭ��Ӧ����ȷ�����ӷ���ʽΪ��2Fe2++2H++H2O2�T2Fe3++2H2O����C����
D����ͨ�����SO2���NaOH��Һ�м���������ˮ��������SO2���ܽ⣩����Ӧ�����ӷ���ʽΪ��HSO3-+Br2+H2O�T3H++2Br-+SO42-����D��ȷ��
��ѡD��
���� ���⿼�������ӷ���ʽ����д����Ŀ�ѶȲ�����ȷ������Ӧ��ʵ��Ϊ���ؼ���ע�����շ�Ӧ�����������������Ӱ�죬����������ѧ���ķ������������Ӧ��������

| A�� | 100gKHCO3��CaCO3�Ļ������е���������ĿΪNA | |
| B�� | ���³�ѹ�£�1 mol�״���ȫȼ������CO2��H2O��ת�Ƶĵ�����ĿΪ6NA | |
| C�� | ��״���£�22.4L���麬�еĹ��ۼ���ĿΪ10NA | |
| D�� | �����£�0.1L0.1mol/L��NH4Cl��Һ�У�NH4+����ĿΪ0.01NA |
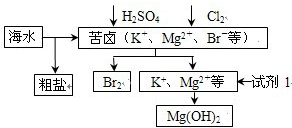
| A�� | �Լ�1����ѡ��ʯ���� | |
| B�� | �ӿ�±����ȡBr2�ķ�Ӧ�����ӷ���ʽΪ��2Br-+Cl2=2 Cl-+Br2 | |
| C�� | ��ҵ�ϣ��������MgOұ������þ�ɼ�С�ܺ� | |
| D�� | ���ξ��ᴿ��������Ʊ��������ƵȲ�Ʒ |
| A�� | pH=1��H2SO4��Һ����H+����ĿΪ2NA | |
| B�� | 1mol Na������O2��Ӧ������Na2O��Na2O2�Ļ�����ʧȥNA������ | |
| C�� | 273K��101kPa�£�14g��ϩ���ϩ������к���̼ԭ����ĿΪ3NA | |
| D�� | 0.2mol C2H6O������һ������0.2NA��̼̼���� |
| A�� | CaO ��CO2 | B�� | NaCl��HCI | C�� | SiC ��SiO2 | D�� | CCl4��I2 |
| A�� | 12C��14CΪ��ͬ���� | |
| B�� | ʯī��C60�Ļ�Ϊͬ�������� | |
| C�� | H2O��D2O��Ϊͬλ�� | |
| D�� | ����������о������Ȼ���-COOH���Ͱ�����-NH2�� |
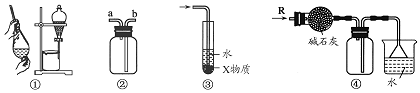
| A�� | װ�âڿ������ռ�H2��NH3��CO2��Cl2��HCl��NO2������ | |
| B�� | װ�â���X��ΪCCl4������������NH3��HCl������ֹ���� | |
| C�� | װ�âٿ����ڷ���C2H5OH��H2O�Ļ���� | |
| D�� | װ�âܿ����ڸ���ռ�NH3�������ն����NH3 |
| A�� | 7.2 g CaO2�����������Ӻ�����������Ϊ0.3 NA | |
| B�� | �ڱ���£�22.4LHF��22.4LC2H4ԭ�Ӹ�����Ϊ1��3 | |
| C�� | ��1 mol NH4NO3����ϡ��ˮ��ʹ��Һ�����ԣ���Һ��NH4+��ĿΪNA | |
| D�� | 0.1 mol H2O2�����к����Թ��ۼ���ĿΪ0.3 NA |