��Ŀ����
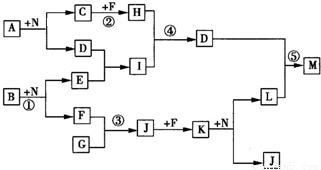
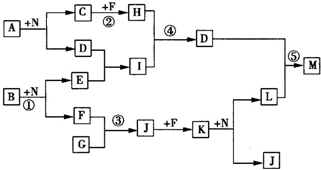 ���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��
���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪������FΪ���ʣ�
����A��B��C��G��H��J��K��N����������Ԫ����ɵĻ������C��GΪͬ����Ԫ���γɵļ���̬�⻯�
����D��E��I��L��M������3��Ԫ����ɵĻ������D�������ԣ�
��������Ӧ�����ɵ�ˮ������ȥ��
��ش��������⣺
��1��д����ѧʽ��B
Na2O2
Na2O2
��INaAlO2
NaAlO2
����2��д��E�ĵ���ʽ��
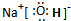
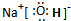
��3����Ӧ�۵Ļ�ѧ����ʽΪ
4NH3+5O2
6H2O+4NO
| ||
| �� |
4NH3+5O2
6H2O+4NO
��
| ||
| �� |
��4�����ͷ�Ӧ�������ɵ�M��ˮ��Һ�����Ե�ԭ��
��������ǿ�������Σ�������ˮ���ʹ��ˮ��Һ�����ԣ�Al3++3H2O?Al��OH��3+3H+
��������ǿ�������Σ�������ˮ���ʹ��ˮ��Һ�����ԣ�Al3++3H2O?Al��OH��3+3H+
�����û�ѧ����ʽ����Ҫ������˵��������5����Ӧ���У�ÿ1.00g C��������F���ã��ָ���25��ʱ�ų�55.6kJ��������д����Ӧ�ڵ��Ȼ�ѧ����ʽ��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6KJ/mol
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6KJ/mol
����Ӧ����KOH�����ʵĻ����п��γ�һ��ԭ��أ����ԭ��صĸ�����ӦʽΪCH4-8e-+10OH-=CO32-+7H2O
CH4-8e-+10OH-=CO32-+7H2O
��������D��������Ԫ����ɵĻ������D�������ԣ�����D������������F�ǵ��ʣ�G���⻯�G��F����J��J��F����K��K��N��Ӧ����L��J��G��J��K��N��ֻ������Ԫ�أ����ת����ϵ���жϸù���Ϊ�����������ٽ��ת���ص����ж�G�ǰ�����F��������J��һ��������K�Ƕ������������ж�B�ǹ������ƣ�E���������ƣ�D������������I��ƫ�����ƣ�A��ˮ��Ӧֻ������ȫ˫ˮ���������������������C�백��Ϊͬ����Ԫ���γɵļ���̬�⻯�Cֻ���Ǽ��飬����A��̼������H�Ƕ�����̼��M����������������ʵ��������������
����⣺D��������Ԫ����ɵĻ������D�������ԣ�����D������������F�ǵ��ʣ�G���⻯�G��F����J��J��F����K��K��N��Ӧ����L��J��G��J��K��N��ֻ������Ԫ�أ����ת����ϵ���жϸù���Ϊ�����������ٽ��ת���ص����ж�G�ǰ�����F��������J��һ��������K�Ƕ������������ж�B�ǹ������ƣ�E���������ƣ�D������������I��ƫ�����ƣ�A��ˮ��Ӧֻ������ȫ˫ˮ���������������������C�백��Ϊͬ����Ԫ���γɵļ���̬�⻯�Cֻ���Ǽ��飬����A��̼������H�Ƕ�����̼��M����������
��1��ͨ�����Ϸ���֪��B�ǹ������ƣ�D��ƫ�����ƣ��仯ѧʽ�ֱ���Na2O2��NaAlO2���ʴ�Ϊ��Na2O2��NaAlO2��
��2��E���������ƣ������ʽΪ��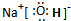 ���ʴ�Ϊ��
���ʴ�Ϊ��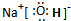 ��
��
��3���ڴ��������������£�������������������һ��������ˮ����Ӧ����ʽΪ��4NH3+5O2
6H2O+4NO���ʴ�Ϊ��4NH3+5O2
6H2O+4NO��
��4��M������������������ǿ�������Σ�������ˮ���ʹ��ˮ��Һ�����ԣ�Al3++3H2O?Al��OH��3+3H+���ʴ�Ϊ����������ǿ�������Σ�������ˮ���ʹ��ˮ��Һ�����ԣ�Al3++3H2O?Al��OH��3+3H+��
��5��ÿ1.00g �������������������ã��ָ���25��ʱ�ų�55.6kJ��������1.00g��������ʵ���=
=
mol����1mol������ȫȼ�շų�������=
=889.6KJ/mol���������Ȼ�ѧ��Ӧ����ʽΪ��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6KJ/mol��
�����ϼ���ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ��������缫��ӦʽΪ��CH4-8e-+10OH-=CO32-+7H2O��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6KJ/mol��CH4-8e-+10OH-=CO32-+7H2O��
��1��ͨ�����Ϸ���֪��B�ǹ������ƣ�D��ƫ�����ƣ��仯ѧʽ�ֱ���Na2O2��NaAlO2���ʴ�Ϊ��Na2O2��NaAlO2��
��2��E���������ƣ������ʽΪ��
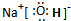 ���ʴ�Ϊ��
���ʴ�Ϊ��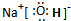 ��
����3���ڴ��������������£�������������������һ��������ˮ����Ӧ����ʽΪ��4NH3+5O2
| ||
| �� |
| ||
| �� |
��4��M������������������ǿ�������Σ�������ˮ���ʹ��ˮ��Һ�����ԣ�Al3++3H2O?Al��OH��3+3H+���ʴ�Ϊ����������ǿ�������Σ�������ˮ���ʹ��ˮ��Һ�����ԣ�Al3++3H2O?Al��OH��3+3H+��
��5��ÿ1.00g �������������������ã��ָ���25��ʱ�ų�55.6kJ��������1.00g��������ʵ���=
| 1.00g |
| 16g/mol |
| 1 |
| 16 |
| 55.6KJ | ||
|
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6KJ/mol��
�����ϼ���ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ��������缫��ӦʽΪ��CH4-8e-+10OH-=CO32-+7H2O��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6KJ/mol��CH4-8e-+10OH-=CO32-+7H2O��
����������ͼ���Ǹ߿��ij������ͣ�����ת��Ϊ���������ؿ���˫���Ǵ������һ�����㣬��ȷ���ʵ����ʼ�������ѧ��Ӧ�ǽⱾ��ؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
�����Ŀ
 ���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��
���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��