��Ŀ����
��ͼ1-5-14��ʾװ�ý���ʵ�飬��A��μ�����ƿ�С�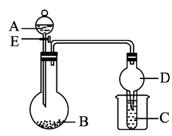
ͼ1-5-14
(1)��AΪŨ���BΪ�������ڽ���Ԫ�ص�Ƭ״���ʣ����ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ���й۲쵽��Һ��ɫ����B��_____________(д��ѧʽ)��B��ŨH2SO4��Ӧ�Ļ�ѧ����ʽΪ_____________����Ӧ�����ձ��м����ˮ���ֿɹ۲쵽�Թ�C�е�����Ϊ__________________________��
(2)��BΪNa2CO3��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ����ǣ�����AӦ���е�������____________________��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�������___________________��
(3)��B����ʯ�ң�ʵ���й۲쵽C��Һ���γɳ�����Ȼ������ܽ⣬����Һǡ�ó���ʱ���ر�E��Ȼ�����ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��_____________ (д����)��C��_____________ (д��ѧʽ)�������ǵĻ��Һ���÷�Ӧ�����ӷ���ʽΪ_____________������D�ڴ�ʵ���е�������__________________________��
����:�����ۺϿ���������Mg��Al��H2SO4��Na2CO3��NH3��H2O���л��ﱽ�ӡ������ǵ�֪ʶ��Ӧ�ã������൱��֪ʶ��Ⱥ�˼ά���������е�(1)�ʣ�B��������ԭ���dz��������ۻ�����(2)�ʣ������ˮ�����ӵ��ܽ������(3)�ʣ�D�����ݻ��ɷ�����(�����ڵ���©��)��
��:(1)Mg Mg+2H2SO4(Ũ)====MgSO4+SO2��+2H2O C��Һ���
(2)���Ա�̼��ǿ ����Һ�����
(3)Ũ��ˮ AgNO3
CH2OH(CHOH)4CHO+2[Ag(NH3)2��++2OH-![]() CH2OH(CHOH)4COO-+
CH2OH(CHOH)4COO-+![]() +2Ag��+3NH3+H2O ��ֹ����
+2Ag��+3NH3+H2O ��ֹ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� �����£���0.10mol?L-1KOH��Һ�ζ�10.00mL 0.10mol?L-1H2C2O4��Һ���õĵζ�������ͼ��ʾ�������Һ������ɿ��ɻ��ǰ����Һ�����֮�ͣ�����ش��������⣺
�����£���0.10mol?L-1KOH��Һ�ζ�10.00mL 0.10mol?L-1H2C2O4��Һ���õĵζ�������ͼ��ʾ�������Һ������ɿ��ɻ��ǰ����Һ�����֮�ͣ�����ش��������⣺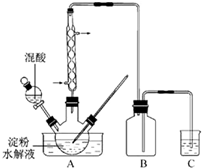 ��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��
��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ�� ���ᡢ5%NaOH��Һ������
���ᡢ5%NaOH��Һ������ ��Һ������
��Һ������ ��Һ������ˮ��
��Һ������ˮ��