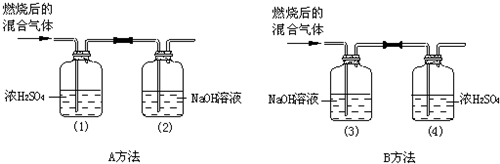
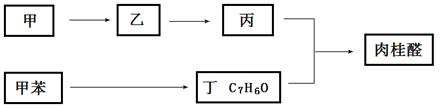
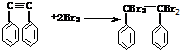��Ŀ����
��ͼ�У�����C��H��O����Ԫ����ɣ�Ԫ�ص�������Ϊ12:3:8���ķе�Ϊ78.5�棬��������H2������ܶ���23�����¶ȿ�����400�����£���Ҫ�����ʵ�顣

��1����ͨ������������½���ʵ���
�ټ������� ������ҩƷ��IJ��������� ��
a.���� b.�ù������Ϲ������ c.��ȼ�ƾ��Ƹ�ͭ˿����
��ʵ�������ȡ�Թ��е���Һ�����Ƶ�Cu(OH)2��ϣ����������ڣ�ʵ������Ϊ
��
������10%��NaOH��Һ��2%��CuSO??4��Һ����ȡ��ʵ�������Լ�Cu(OH)2�������ʵ�������
��
��2����ֹͣͨ������������½���ʵ���
�ٹرջ�����Ϊʹ�׳������뷴Ӧ���У���Ҫ���еIJ�����
��
�ڼ��������뷴Ӧ�ܺ���ͭ��������250~350�������·�������Ļ�ѧ��Ӧ��
���Թ����ռ�����ʵ�����ͬ�IJ�����п�ȼ�����嵥�ʷų����÷�Ӧ��ʾ�˼�
�������ı��ʡ�д��ʵ����з�Ӧ�Ļ�ѧ����ʽ������ϸû�ѧ����ʽ��Ҫ˵��ʵ�������ͨ���������á�
��15�֣���1�����Ҵ���2�֣���cab����acb����2�֣�
���к�ɫ����������2�֣�
��ȡ2mL��10%��NaOH��Һ�����Թ��У��ý�ͷ�ιܵ���4��~6��2%��CuSO4��Һ����
������NaOH��Һ�еμ�����CuSO4��Һ����1�֣��ޡ����������÷֣���2�֣�
��2�������ձ���ע��ˮ����78.5������ˮԡ���ȡ�
| |||||||||||||