��Ŀ����

ijͬѧ����1���ȶ����ˮ��ʵ�飬���������е���Ԫ�ء�ʵ��������£�
��ͼ��ʾ���ڴ��Թ��м���5 mL 1 mol��L��1NaOH��Һ��5 mL 1?�ȶ���(1?�ȶ���ķе�Ϊ77��78 �棬�ܶ�Ϊ0.886 g��cm��3����ȼ)��ˮԡ���ȸ��Թ�10 min���ϣ������Ƽ����¶���70��80 �档ȡ2֧С�Թܣ�������Լ1 mL��2%��AgNO3��3 mol��L��1���ᰴ1��1������ɵĻ����Һ���ý�ͷ�ι���ȡ���Ⱥ���Թ��ڵ��ϲ���Һ�����˴���Һ��μ�������һ֧С�Թ��У�����һ֧δ�Ӵ���Һ��С�Թ�����Һ��ȣ��а�ɫ�Ļ�������֣�˵��1���ȶ�����NaOH��Һ��Ӧ��Cl�����ɣ��Ӷ�֤����1?�ȶ����к�����Ԫ�ء�
��ش�
(1)��д��1?�ȶ���ˮ��Ļ�ѧ����ʽ��
________________________________________________________________________��
(2)�Թ��Ϸ��IJ������ܵ�����Ϊ__________________��
(3)����Cl��ʱ���������ữAgNO3��Һ��Ŀ����
________________________________________________________________________��
(4)��װ�����л���ѧʵ���г��õ�һ��װ�ã�ʵ�����У�����ʹ������װ���Ʊ�����________(�����)��
A���������������������������� B����ϩ
C���������� D���屽
(1)CH3CH2CH2CH2Cl��NaOH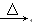 CH3CH2CH2CH2OH��NaCl
CH3CH2CH2CH2OH��NaCl
(2)���������������������ͨ
(3)�ų����������ӵĸ��š�(4)A
��������
���������(1)��1���ȶ���ˮ������з���������Ӧ������ˮ������1?������HCl������HCl��NaOH��Һ��Ӧ���ٽ���1?�ȶ����ˮ�⡣
(2)����1?�ȶ���ķе�ֻ��77��78 �棬�ڼ���ʱ���ӷ������ֲ����ܷ⣬����װ����Ч�ؽ����������⡣һ���棬���������������ͨ�������ڳ���Σ�գ�������ʱ��ҩƷ���ϻӷ��Ĺ������������������������±��Һ�壬�������Թ��У������μӷ�Ӧ��
(3)�������ữAgNO3��Һ��Ŀ�ģ����кʹ���Һ�е�NaOH����ֹNaOH��AgNO3��Ӧ���ɳ�����Ӱ��ʵ��Ч����
(4)ʵ������ȡ������ʱ����50��60 �������½��У�����Ӧ������е����ᡢ�������ӷ���������ʵ���������ƣ�����ʹ������װ�á�����ϩ����170 �������½��У�������ˮԡ���ȣ�����������ʱֱ�Ӽ��ȼ��ɡ����屽���ü��ȡ�
���㣺����±������±��ԭ�Ӽ�����й�ʵ��̽��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ����ע�ض�ѧ������֪ʶѵ���ͼ����ͬʱ�����ض�ѧ��ʵ����������������ͷ����뼼�ɵ�ָ����ѵ�������������ѧ����ʵ�����������Ӧ������������ѧ����ѧ������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������
