��Ŀ����
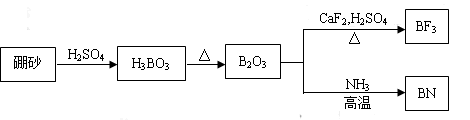
 ����һ����Ҫ�ķǽ���Ԫ�أ����������ͨ����þ��Mg2B2O5?H2O����ȡ��
����һ����Ҫ�ķǽ���Ԫ�أ����������ͨ����þ��Mg2B2O5?H2O����ȡ���ڢٲ�������þ����Ũ��NaOH��Һ�ܽ⣬���˵�NaBO2��Һ��
�ڢڲ�����NaBO2Ũ����ͨ��CO2���ڼ�ȣ��ᾧ������ɰNa2B4O7?10H2O��
�ڢ۲�������ɰ����ˮ����H2SO4������ȣ����˵�H3BO3���壮
�ڢܲ�����������ʹ֮��ˮ���������
�ڢݲ�����ˮ������þ��ԭ���ôֵ�������ش��������⣺
��1���ڢٲ��͵ڢݲ��Ļ�ѧ����ʽ�ֱ�Ϊ
��2�����ƵõĴ�����һ�������·�Ӧȫ������BI3��Ȼ��BI3�ȷֽ���Եõ������ĵ�����0.20g�����Ƴɵ�BI3�ֽ�õ���I2ȫ�����ռ����� 2.00mol/LNa2S2O3��Һ�ζ�����ȥ27.00mL Na2S2O3��Һ����֪��I2+2S2O32-=2I-+S4O62-����
��Na2S2O3��Һ�ɼ��ԣ������ӷ���ʽ������ԭ��
�ڴ�������ĺ���Ϊ
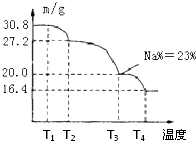
��3�������Ƶõ���ɰ���壨Na2B4O7?10H2O�������Ʊ��������ƣ�����һ��������Ư�������㷺Ӧ����ϴ�·ۡ�Ư�ۡ�ϴ�Ӽ��У���֪��Ʒ�������ƾ����и�Ԫ�ص����ʵ���֮��Ϊn��Na����n��B����n��H����n��O��=1��1��n��7�����ƵõĴ�Ʒ��Ʒ��70�����ϼ��Ƚ���ʧȥ�ᾧˮ����ô�Ʒ�������¶ȵı仯����ͼ��ʾ����T3ʱ���þ���Ļ�ѧʽΪ��
��������1�����ݵڢٲ�ΪMg2B2O5?H2O������������Һ�ķ�Ӧ���ڢݲ�ΪB2O3��Mg�ķ�Ӧд����Ӧ�ķ���ʽ��
��2���ٸ��ݼ�����Һʹ�ü�ʽ�ζ�����ɣ����ݵ�����ⵥ����ʾ��ɫ�жϣ����ݷ�Ӧ��������Һ����ɫ��ʧ������
�ڸ��ݷ�Ӧ����ʽ���ζ����ݼ������������ĺ�����
��3�����ݸ�Ԫ�����ʵ������Լ�ͼ�и����ݼ������Ԫ�ص�ԭ�Ӹ����ȣ�д����ѧʽ��
��2���ٸ��ݼ�����Һʹ�ü�ʽ�ζ�����ɣ����ݵ�����ⵥ����ʾ��ɫ�жϣ����ݷ�Ӧ��������Һ����ɫ��ʧ������
�ڸ��ݷ�Ӧ����ʽ���ζ����ݼ������������ĺ�����
��3�����ݸ�Ԫ�����ʵ������Լ�ͼ�и����ݼ������Ԫ�ص�ԭ�Ӹ����ȣ�д����ѧʽ��
�����1���ڢٲ�ΪMg2B2O5?H2O������������Һ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��Mg2B2O5?H2O+2NaOH=2NaBO2+2Mg��OH��2�����ڢݲ�ΪB2O3��Mg��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��B2O3+3Mg=3MgO+2B��
�ʴ�Ϊ��Mg2B2O5?H2O+2NaOH=2NaBO2+2Mg��OH��2����B2O3+3Mg=3MgO+2B��
��2����Na2S2O3��Һ�ɼ��ԣ�S2O32-����ˮ����Һ��ʾ���ԣ���Ӧ�����ӷ���ʽΪ��S2O32-+H2O?HS2O3-+OH-���ζ��������еⵥ�ʲ��룬����ʹ�õ�����Һ��Ϊָʾ�����ζ�����ʱ����ƿ����Һ��ɫ��ȥ��30s�ڲ���ɫ��
�ʴ�Ϊ��S2O32-+H2O?HS2O3-+OH-��������Һ����ƿ����Һ��ɫ��ȥ��30s�ڲ���ɫ��
����������Ƶ����ʵ���Ϊ��2.00mol/L��0.027L=0.054mol�����ݹ�ϵʽ��B��BI3��
I2��3S2O32-��n��B��=
n��S2O32-��=0.018mol��
�������Ϊ��10.8g/mol��0.018mol=0.1944g����������ĺ���Ϊ��
��100%=97.2%��
�ʴ�Ϊ��97.2%��
��3��������̣�T3ʱ��n��Na��=
=0.2mol
��30.8g��Ʒ�У�����n��Na����n��B����n��H����n��O��=1��1��n��7=0.2��0.2��0.2n��1.4��
��Ʒ������Ϊ��0.2��23+0.2��11+0.2n+1.4��16=30.8
��ã�n=8����ԭ����Ϊ��NaBO3?4H2O��
T3ʱ���þ�������Ԫ�ص���������������ᾧˮ��Ŀ��
=0.23�����n=1��
����T3ʱ���þ���Ļ�ѧʽΪNaBO3?H2O��
�ʴ�Ϊ��NaBO3?H2O��
�ʴ�Ϊ��Mg2B2O5?H2O+2NaOH=2NaBO2+2Mg��OH��2����B2O3+3Mg=3MgO+2B��
��2����Na2S2O3��Һ�ɼ��ԣ�S2O32-����ˮ����Һ��ʾ���ԣ���Ӧ�����ӷ���ʽΪ��S2O32-+H2O?HS2O3-+OH-���ζ��������еⵥ�ʲ��룬����ʹ�õ�����Һ��Ϊָʾ�����ζ�����ʱ����ƿ����Һ��ɫ��ȥ��30s�ڲ���ɫ��
�ʴ�Ϊ��S2O32-+H2O?HS2O3-+OH-��������Һ����ƿ����Һ��ɫ��ȥ��30s�ڲ���ɫ��
����������Ƶ����ʵ���Ϊ��2.00mol/L��0.027L=0.054mol�����ݹ�ϵʽ��B��BI3��
| 3 |
| 2 |
| 1 |
| 3 |
�������Ϊ��10.8g/mol��0.018mol=0.1944g����������ĺ���Ϊ��
| 0.1944g |
| 2.00g |
�ʴ�Ϊ��97.2%��
��3��������̣�T3ʱ��n��Na��=
| 20.0g��23% |
| 23g/mol |
��30.8g��Ʒ�У�����n��Na����n��B����n��H����n��O��=1��1��n��7=0.2��0.2��0.2n��1.4��
��Ʒ������Ϊ��0.2��23+0.2��11+0.2n+1.4��16=30.8
��ã�n=8����ԭ����Ϊ��NaBO3?4H2O��
T3ʱ���þ�������Ԫ�ص���������������ᾧˮ��Ŀ��
| 23 |
| 23+11+48+18n |
����T3ʱ���þ���Ļ�ѧʽΪNaBO3?H2O��
�ʴ�Ϊ��NaBO3?H2O��
���������⿼���������ˮ�⡢���ӻ�ѧʽ�ļ��㣬��Ŀ�Ѷ��Դ������ѵ㣬����ʱҪ���������Ŀ����������ϵ�ͱ������ݣ�����������ϵ�����������ĸ�����ٽ��⣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��10�֣�
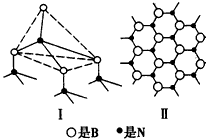
I.��6�֣�������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
��ش��������⣺
(1)��B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������ ��
��
(2) BN��BԪ�صĻ��ϼ�Ϊ ��
II. ��4�֣���ҵ����ȡ����ʯ(Na3AlF6)�Ļ�ѧ����ʽ���£�
2Al(OH)3+12HF+3Na2CO3=2Na3AlF6+3CO2��+9H2O
�����������գ�
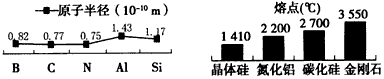
(3����Ӧ����������Ԫ����Ԫ�����ڱ���λ�����ڣ��������ж����ǵĽ����Ի�ǽ�����ǿ������ (ѡ����ĸ����
| A����̬�⻯����ȶ��� | B������������Ӧˮ��������� |
| C��������������Ӧ������ | D��������ͬŨ���ᷢ����Ӧ�Ŀ��� |
 ����COCl2�����ں���_____���м���Cԭ�ӵ��ӻ���ʽΪ____��
����COCl2�����ں���_____���м���Cԭ�ӵ��ӻ���ʽΪ____��