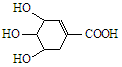
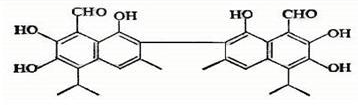
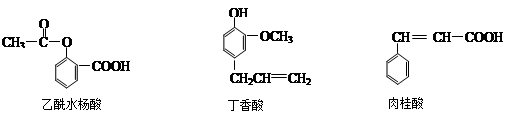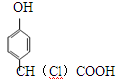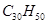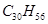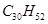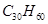��Ŀ����
��1������ʽΪC5H12O���ڴ����ܴ���������ȩ���л����� �֣�
��2��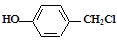 ��NaOHˮ��Һ���ȣ����ɵ��л���Ľṹ��ʽΪ________��
��NaOHˮ��Һ���ȣ����ɵ��л���Ľṹ��ʽΪ________��
��3��ij�л���ķ���ʽΪC6H12���������������̼ԭ��һ������ͬһƽ�棬����л���Ľṹ��ʽΪ_______________�������л�����һ����ʽ�칹�壬�����������ӳ�����2-�����飬����л���Ľṹ��ʽΪ_______________��
��4���л���C6H5C��CCH2CBr3�����У���ͬһ��ֱ���ϵ�ԭ�������__________����
��5���ڳ���ı�������Һ��ͨ��CO2���壬��Һ���ǣ��䷴Ӧ����ʽ�ǣ�
________________________________________________________________________��
��2��
 ��NaOHˮ��Һ���ȣ����ɵ��л���Ľṹ��ʽΪ________��
��NaOHˮ��Һ���ȣ����ɵ��л���Ľṹ��ʽΪ________����3��ij�л���ķ���ʽΪC6H12���������������̼ԭ��һ������ͬһƽ�棬����л���Ľṹ��ʽΪ_______________�������л�����һ����ʽ�칹�壬�����������ӳ�����2-�����飬����л���Ľṹ��ʽΪ_______________��
��4���л���C6H5C��CCH2CBr3�����У���ͬһ��ֱ���ϵ�ԭ�������__________����
��5���ڳ���ı�������Һ��ͨ��CO2���壬��Һ���ǣ��䷴Ӧ����ʽ�ǣ�
________________________________________________________________________��
(13��) (1) 4 (2) 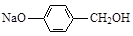 (3) (CH3)2C=C(CH3)2
(3) (CH3)2C=C(CH3)2 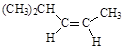
(4) 6����2�֣� (5)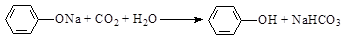 ��3�֣�
��3�֣�
 (3) (CH3)2C=C(CH3)2
(3) (CH3)2C=C(CH3)2 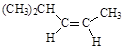
(4) 6����2�֣� (5)
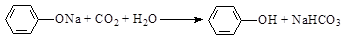 ��3�֣�
��3�֣������������1���������������������ǣ����ǻ����ӵ�̼ԭ���Ϻ�����ԭ�ӣ����Է���ʽΪC5H12O���ڴ����ܴ���������ȩ���л�����CH3CH2CH2CH2CH2OH��CH3CH2CH(CH3(CH2OH��(CH3)2CHCH2CH2OH��(CH3)3CCH2OH��������4�֡�
��2���л����к��з��ǻ�����ԭ�ӣ����ܺ�����������Һ��Ӧ����������Ľṹ��ʽ��
 ��
����3�����ں�̼̼˫��������̼ԭ��һ��λ��ͬһ��ƽ���ϣ����Է����������л���Ľṹ��ʽ��(CH3)2C=C(CH3)2������2��������Ľṹ��ʽ(CH3)2CHCH2CH2CH3��֪��Ҫ��������Ӧ��ϩ������˳���칹�壬���ϩ���Ľṹ��ʽһ����
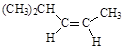 ��
����4�����ڱ�����ƽ���������νṹ����̼̼������ֱ���ͽṹ�����л���C6H5C��CCH2CBr3�����У���ͬһ��ֱ���ϵ�ԭ�������6����
��5�����ӵ�����ǿ��̼�����Ƶģ�������̼��ģ���÷�Ӧ�Ļ�ѧ����ʽ��
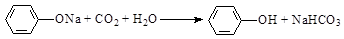 ��
�������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿����ۺ���ǿ����ע�ض�ѧ������֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ�������������ѧ����Ӧ�������ʹ���Ч�ʣ�Ҳ����������ѧ��֪ʶ��Ǩ������������Ĺؼ��Ǽ�ס���������ŵĽṹ�������Լ�������֮����ת����Ȼ��������������ü��ɡ�

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
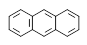 ��һ��ȡ������ͬ���칹���������
��һ��ȡ������ͬ���칹���������
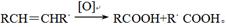 д��C��ǿ���������������ɵ��л�������Ľṹ��ʽ ��
д��C��ǿ���������������ɵ��л�������Ľṹ��ʽ ��