��Ŀ����
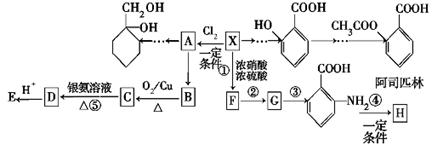
����������ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ�������������H�ĺϳ�·�ߣ�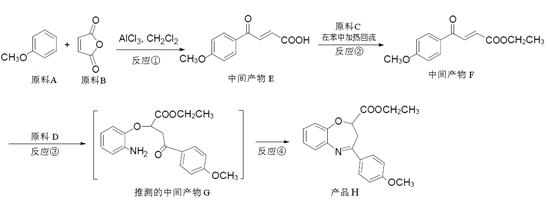
��1��ԭ��A��ͬ���칹���У����б����Һ˴Ź�����������5����Ľṹ��ʽΪ ��
д�������ʴ�������Ӧ�Ļ�ѧ����ʽ ��
��2���ڵķ�Ӧ������ ��ԭ��D�к��еĹ����������� �� ��
��3��д�����������������м����F��ͬ���칹��Ľṹ��ʽ ��
(i) �ܷ���������Ӧ��
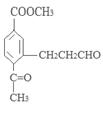
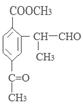
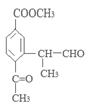
(ii) �����к�����ȡ���ı����ṹ����������ȡ�����ǣ���COOCH3�� ���Ҷ��ߴ��ڶ�λ��
���Ҷ��ߴ��ڶ�λ��
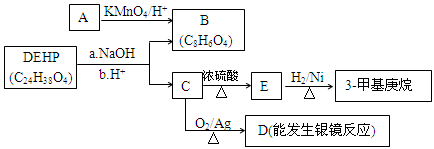
��4��ԭ��B�������������������������ᣨHOOC-CH=CH-COOH�����������������ԭ��CH2OH-CH=CH-CH2OH�ϳ�������ĺϳ�·�ߡ��ϳ�·������ͼʾ�����£�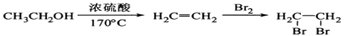
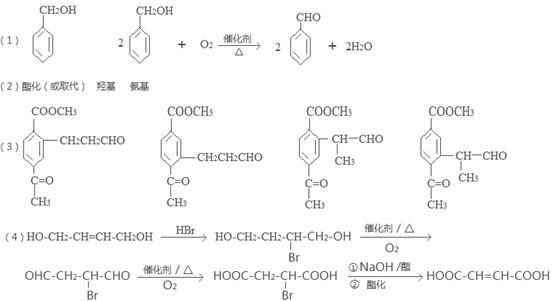
���������������1��ԭ��A�ķ���ʽΪC7H8O�����ĺ��б����Һ˴Ź�����������5�����ͬ���칹��ṹ��ʽΪ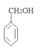 ��д�������ʴ�������Ӧ�Ļ�ѧ����ʽ
��д�������ʴ�������Ӧ�Ļ�ѧ����ʽ ����2����Ӧ�ڵĻ�ѧ��Ӧ����Ϊ������Ӧ����ȡ����Ӧ����ԭ��D�ڰ��������к��еĹ�����Ϊ�ǻ�����������3��F�ķ���Ҫ���ͬ���칹��
����2����Ӧ�ڵĻ�ѧ��Ӧ����Ϊ������Ӧ����ȡ����Ӧ����ԭ��D�ڰ��������к��еĹ�����Ϊ�ǻ�����������3��F�ķ���Ҫ���ͬ���칹�� ��
�� ��
�� ��
��
��4����ԭ��CH2OH-CH=CH-CH2OH�ϳ�������ĺϳ�·��Ϊ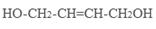 +HBr
+HBr
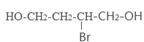 ��
��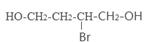 +O2
+O2 
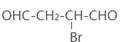 ��
��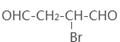 +O2
+O2
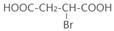 ��
��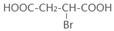 +NaOH
+NaOH
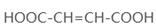 +NaBr+H2O��
+NaBr+H2O��
���㣺�����л�������š��ṹ��ʽ��ͬ���칹�����д���ϳɵ�֪ʶ��

 һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�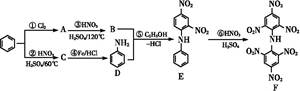

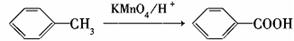
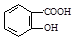 �ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯����,��˴Ź�������ͼ�з����֮��Ϊ1:2:2:1���������������������ͬ���칹��Ľṹ��ʽ�� ��
�ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯����,��˴Ź�������ͼ�з����֮��Ϊ1:2:2:1���������������������ͬ���칹��Ľṹ��ʽ�� ��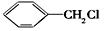 ����������ϳ�
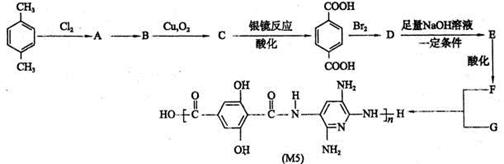
����������ϳ�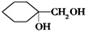 ��������ķ�����������4���������磺
��������ķ�����������4���������磺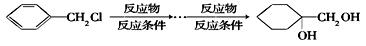
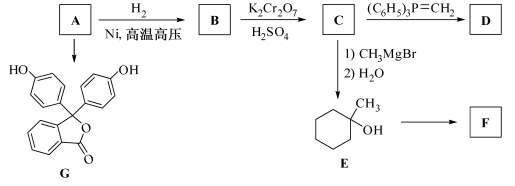
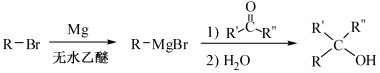
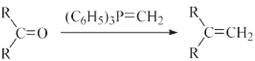 (R��ʾ������R���R���ʾ��������)
(R��ʾ������R���R���ʾ��������) �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�