��Ŀ����
ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W������Ԫ�ص�ԭ������֮��Ϊ32�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ�Y��Z�������ڣ�Z��Wλ��ͬ���塣
��1��XԪ���� �������ƣ� ,W�����ڱ��е�λ�� ��
��2��X��Y �γɻ�����ĵ���ʽΪ ��X��W��ɵĻ������д��� ��������ӡ������ۡ�����
��3����д��ʵ�����Ʊ�YX3�Ļ�ѧ����ʽ�� ��
�ڹ�ҵ��Ҳ����ѡ����ʵ���������YX3�ĺϳɣ�����֪�ڸ�������ÿ����2molYX3����ʱ�ų�
92.4kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��4����X��Y��Z��W����Ԫ����ɵ�һ�����ӻ�����A����֪1mol A��������NaOHŨ��Һ��Ӧ���ɱ�״����44.8L���塣��A�������� ��
��1��XԪ���� �������ƣ� ,W�����ڱ��е�λ�� ��
��2��X��Y �γɻ�����ĵ���ʽΪ ��X��W��ɵĻ������д��� ��������ӡ������ۡ�����
��3����д��ʵ�����Ʊ�YX3�Ļ�ѧ����ʽ�� ��
�ڹ�ҵ��Ҳ����ѡ����ʵ���������YX3�ĺϳɣ�����֪�ڸ�������ÿ����2molYX3����ʱ�ų�
92.4kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��4����X��Y��Z��W����Ԫ����ɵ�һ�����ӻ�����A����֪1mol A��������NaOHŨ��Һ��Ӧ���ɱ�״����44.8L���塣��A�������� ��
��1��H �������ڵڢ�A��
��2��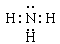 ����
����
��3����2NH4Cl + Ca��OH��2 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O
��N2(g)+3H2(g) 2NH3(g) ��H=-92.4KJ/mol
2NH3(g) ��H=-92.4KJ/mol
��4������炙��������
��2��
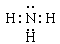 ����
������3����2NH4Cl + Ca��OH��2
 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O��N2(g)+3H2(g)
 2NH3(g) ��H=-92.4KJ/mol
2NH3(g) ��H=-92.4KJ/mol��4������炙��������
���������ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ���XΪHԪ�أ�Z��Wλ��ͬ���壬��Z��ԭ������Ϊx����W��ԭ������Ϊx+8��Y��Z�������ڣ�Y��ԭ������Ϊx-1��������Ԫ�ص�ԭ������֮��Ϊ32����1+��x-1��+x+��x+8����32�����x��8����YΪNԪ�أ�ZΪOԪ�أ�WΪSԪ�ء�
��1��������������֪��XԪ����HԪ�أ�W����Ԫ�أ�λ��Ԫ�����ڱ��ĵ������ڵڢ�A�壬�ʴ�Ϊ��H���������ڵڢ�A�塣
��2��X��Y �γɵĻ������ǰ��������м��Լ��Ĺ��ۻ��������ʽΪ
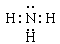 ��X��W��ɵĻ�������H2S��������H��S֮���Թ��õ��Ӷ��γɻ�������Թ��ۼ���ϣ��ʴ�Ϊ�����ۣ�
��X��W��ɵĻ�������H2S��������H��S֮���Թ��õ��Ӷ��γɻ�������Թ��ۼ���ϣ��ʴ�Ϊ�����ۣ�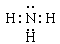 ��
����3����ʵ�����Ʊ�����������ʯ�Һ��Ȼ�識��ȣ���Ӧ�Ļ�ѧ����ʽΪ2NH4Cl + Ca��OH��2
 CaCl2 + 2NH3��+ 2H2O��
CaCl2 + 2NH3��+ 2H2O����ÿ����2mol��������ʱ�ų�92.4kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2(g)
 2NH3(g) ��H����92.4KJ/mol��
2NH3(g) ��H����92.4KJ/mol����4��X��Y��Z��W����Ԫ����ɵ�һ�����ӻ������е�������ΪNH4+��������Ϊ��������ӡ�����������ӡ�����������ӡ�������������ӣ���1mol A��������NaOHŨ��Һ��Ӧ���ɱ�״����44.8L���壬��1molA�к���2molNH4+����AΪ����炙�������李�

��ϰ��ϵ�д�
�����Ŀ
 �� �Իش�
�� �Իش�
