��Ŀ����
��8�֣��л���A 0.02 mol ����������ȫȼ������4.4g CO2��2.16g H2O���������������ɡ��Իش��������⣺
������˵������ȷ���� ����д��ţ�
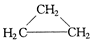
����AΪ����������һ±����ֻ��һ�ֽṹ�������Ľṹ��ʽΪ�� ����ϵͳ������Ϊ�� ���������� ��ͬ���칹�塣
����AΪһԪ�����ڿ����в��ܱ�Cu����������Ӧ��ȩ����A�Ľṹ��ʽΪ �������ƣ�ϵͳ������Ϊ
1mol��A�������Ʒ�Ӧ��������H2�����Ϊ L��STP��
������˵������ȷ���� ����д��ţ�
| A���û�����϶���OԪ�� | B���û�������ܲ���OԪ�� |
| C���û�����϶����ܺ�Na��Ӧ | D���÷�����C:H�ĸ�����Ϊ5:12 |
����AΪһԪ�����ڿ����в��ܱ�Cu����������Ӧ��ȩ����A�Ľṹ��ʽΪ �������ƣ�ϵͳ������Ϊ
1mol��A�������Ʒ�Ӧ��������H2�����Ϊ L��STP��
��8�֣���1��BD��2��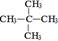 2,2-�������飬3��
2,2-�������飬3��
��3��(CH3)2C(OH)CH2CH3 2��-2-������11.2
 2,2-�������飬3��
2,2-�������飬3����3��(CH3)2C(OH)CH2CH3 2��-2-������11.2
��

��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ
 ��CH3�� �� ��
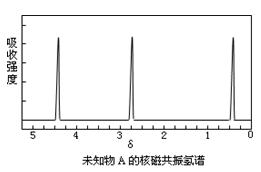
��CH3�� �� �� λ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ���źţ������ݷ�ֵ���źţ�����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ��ѣ�Cl�DCH2�DO�DCH3����������ԭ�ӣ�����ͼ�������ⶨ���л���A�ĺ˴Ź�������ͼ������ͼ����A�Ľṹ��ʽΪ ��
λ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ���źţ������ݷ�ֵ���źţ�����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ��ѣ�Cl�DCH2�DO�DCH3����������ԭ�ӣ�����ͼ�������ⶨ���л���A�ĺ˴Ź�������ͼ������ͼ����A�Ľṹ��ʽΪ ��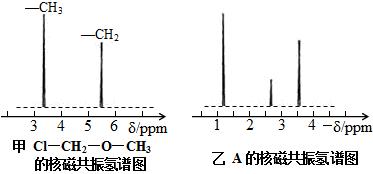

 ��
��
 ��
��
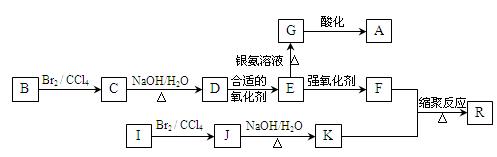
 Һ�������������ϩ
Һ�������������ϩ

