��Ŀ����
����ǿ������ʾ���Ũ���йء��ش��������⣺
��1��ijѧϰС����15 mol/LŨ��������100 mL3 mol/Lϡ���ᡣ
��������Ҫ����Ͳ��ȡ15 mol/LŨ����_______mL��
������ͼ��ʾ�����������ƹ����в����õ���_________������ţ���
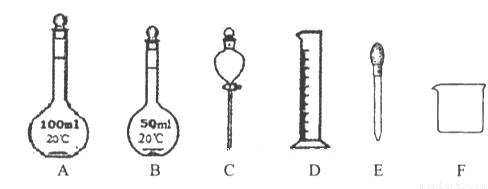
��ͼ�����е������⣬����������Һ�����õ��IJ���������___________��
������ʵ������У�����ȷ����_________����д��ţ���
A��ʹ������ƿǰ��������Ƿ�©ˮ��
B������ʱҺ�泬���̶��ߣ������Һ��Ӧ�ý�ͷ�ι�������
C��������Һʱ������Ͳ��ȡŨ����ֱ�ӵ�������ƿ�У�Ȼ�������ˮ���ݡ�
D�����ݺ�Ǻ�ƿ����������ƿ�������µߵ���ҡ�ȡ�
������Ͳ��ȡŨ���ᣬ����ʱ��������Ͳ����������Һ��Ũ��_________������ƫ�ߡ�ƫ�͡���Ӱ��������
��2����ͭ��Ũ�����ڼ��������·�Ӧ��Ũ������ֳ�����������___________��
��MnO2��Ũ�����ڼ��������·�Ӧ�����ӷ���ʽΪ__________��
���ֱ���Ũ�����ϡ������ȡ��ͬ����Cu(NO3)2���壬����Ũ�����ϡ��������ʵ���֮����__________��
��1����20.0mL ��BC ������ ��BC ��ƫ��
��2����ǿ�����Ժ����� ��2Cl- + 4H+ + MnO2 Mn2+ + Cl2��+ 2H2O ��3:2
Mn2+ + Cl2��+ 2H2O ��3:2
��������
�����������1����С�⿼��һ�����ʵ���Ũ����Һ�����ơ�������ϡ�Ͷ���c1V1=c2V2���㣻������Ũ��������Ϊx��15 mol/Lx=100 mL��3 mol/L�����x=20.0mL����һ�����ʵ���Ũ����Һ�����ƵIJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ɲ��趨�����������õ���Ͳ���ܽ��õ��ձ��Ͳ���������Һ�õ�����ƿ������100mL��ҺӦ��100mL����ƿ�������õ���ͷ�ιܣ������ƹ����в����õ���50mL����ƿ�ͷ�Һ©����ѡBC�������õ��IJ��������Dz���������A��ʹ������ƿǰ��������Ƿ�©ˮ����ȷ��B������ʱҺ�泬���̶��ߣ�Ӧ�������ƣ�����C��������Һʱ������Ͳ��ȡŨ���������ձ����ܽ⣬��ȴ�����º��ٵ�������ƿ�У�Ȼ�������ˮ���ݣ�����D�����ݺ�Ǻ�ƿ����������ƿ�������µߵ���ҡ�ȣ���ȷ��ѡBC��������Ͳ��ȡŨ���ᣬ����ʱ��������Ͳ������ȡ��Ũ�������ƫС����������Һ��Ũ��ƫ�ͣ���2����ͭ��Ũ�����ڼ��������·�Ӧ�Ļ�ѧ����ʽΪ2H2SO4(Ũ)+Cu CuSO4+SO2��+2H2O��Ũ������ֳ�����������ǿ�����Ժ����ԣ���MnO2��Ũ�����ڼ��������·�Ӧ�����ɶ��Ȼ��̡�������ˮ�����ӷ���ʽΪ2Cl- + 4H+ + MnO2
CuSO4+SO2��+2H2O��Ũ������ֳ�����������ǿ�����Ժ����ԣ���MnO2��Ũ�����ڼ��������·�Ӧ�����ɶ��Ȼ��̡�������ˮ�����ӷ���ʽΪ2Cl- + 4H+ + MnO2 Mn2+ + Cl2��+ 2H2O��������ͭ��Ũ��ϡ���ᷴӦ�Ļ�ѧ����ʽ�жϣ��ֱ���Ũ�����ϡ������ȡ��ͬ����Cu(NO3)2���壬����Ũ�����ϡ��������ʵ���֮����3:2��
Mn2+ + Cl2��+ 2H2O��������ͭ��Ũ��ϡ���ᷴӦ�Ļ�ѧ����ʽ�жϣ��ֱ���Ũ�����ϡ������ȡ��ͬ����Cu(NO3)2���壬����Ũ�����ϡ��������ʵ���֮����3:2��
���㣺����һ�����ʵ���Ũ����Һ�����ơ�Ũ�������������ʵȡ�
