��Ŀ����
��10�֣�Ϊ�˲ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȣ�ij��ѧ�С������˶���ʵ�鷽����ȷ��ȡW g������Ʒ�����250mL��Һ���ֳ��������ʵ�飺
���飺��ȡ25.00mL������Һ�����������������ữ��BaCl2��Һ�����ˡ�ϴ�Ӻ���������Ƶó���������Ϊm1 g
���飺��ȡ25.00mL������Һ�����������������ữ��Ba(NO3)2��Һ�����ˡ�ϴ�Ӻ�����������أ�������Ϊm2 g
���飺��ȡ25.00mL������Һ����a mol/L ������KMnO4��Һ���еζ��� ����KMnO4��Һb mL��
����KMnO4��Һb mL��
��1�� ����250mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ����������ιܺ� ��
��2�� �ڱ���ʵ���еζ�ʱ�Ƿ���Ҫѡ��ָʾ��? (���Ҫ������Ҫ��)���ζ��յ����ɫ�仯�� ��
��3�� �ñ����ʵ�����ݣ�����Na2SO3�Ĵ��� ��
��4�� ʵ���з��֣�����ͬѧ�ⶨ��Na2SO3���ȱȼ���ͱ���ͬѧ�Ľ����Ҫ�͡��Է����������������ԭ�� ��
��1��������ƽ��250mL������ƿ��2�֣�
��2������Ҫ����ɫ����ɫ����4�֣�
��3�� ��2�֣�
��2�֣�
��4�������ṩH+��Ba(NO3)2�ṩNO3- �γ�ϡHNO3����һ����SO32-������SO42-����Ba2+�������BaSO4��������2�֣�
����

��19�֣�����������̵���Ҫ������ͳ��õ���������������ʵ������ģ�ҵ�������̿��Ʊ�������ص�����ͼ��
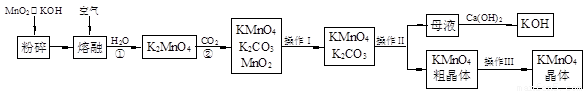
��1�������������Ϊ �������������Ϊ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ����ҵ��������ԭ������KMnO4�������ʽϵͣ��Ϻõ��Ʊ������ǵ�ⷨ����Pt��������Fe��������K2MnO4Ϊ���Һ�������ĵ缫��ӦʽΪ ��
��3��KMnO4��һ�ֽ��ȶ��Ļ�������չ��KMnO4��Һ�ķֽ��д����ã�����MnO2��KOH��O2���� MnO2Ҳ�Ǹ÷ֽⷴӦ��һ�ִ������������һ��ʵ�鷽������֤MnO2�Ը÷ֽⷴӦ���д��ԣ� ��
��4��KMnO4�����Խ����е�ǿ�����Թ㷺Ӧ���ڷ�����ѧ�С�
���磺2KMnO4+3H2SO4+5Na2SO35Na2SO4+K2SO4+2MnSO4+3H2O��ijͬѧ��KMnO4�ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȡ�����ȷ��ȡ6.3 g Na2SO3������Ʒ�����500 mL��Һ��ȡ25.00 mL������Һ������ƿ�У���0.01000 mol/L ������KMnO4��Һ���еζ����ζ�������±���ʾ��
|
�ζ�����[��Դ:][��Դ:Z&xx&k.Com] |
������Һ�����/mL[��Դ:ѧ#��#��Z#X#X#K] |
����Һ�����[��Դ:] |
|
|
�ζ�ǰ�̶�/mL |
�ζ���̶�/mL |
||
|
1 |
25.00 mL |
0.02 |
24.01 |
|
2 |
25.00 mL |
0.70 |
24.71 |
|
3 |
25.00 mL |
0.20 |
24.20 |
������500 mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ�������������ͷ�ιܡ�ҩ�� �� ��
���жϵζ��յ�������� ��
�����в����ᵼ�²ⶨ���ƫ�ߵ���
A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ���
B���ζ�ǰ��ƿδ����
C���ζ�ǰ�ζ��ܼ��첿��������
D���۲����ʱ���ζ�ǰ���ӣ��ζ�����
��������ʵ�����ݣ�����Na2SO3�Ĵ���Ϊ ��
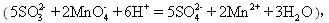 ����KMnO4��Һb mL��
����KMnO4��Һb mL��