��Ŀ����
ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽᣨ���ڳ����£�����֪����ƽ�ⳣ����CH 3COOH��H2CO3��C6H5OH ��HCO
3COOH��H2CO3��C6H5OH ��HCO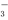 �����в���ȷ����
�����в���ȷ����
A��pH��ȵ�������Һ��a��CH3COONab��C6H5ONac��NaHCO3d��NaOH����������Һ�����ʵ����ʵ���Ũ����С����˳��Ϊ��d<b<c<a
B��pH=8.3��NaHCO3��Һ��c(Na��)��c(HCO3��)��c(CO32��)��c(H2CO3 )
)
C��pH��2��һԪ�� ��pH��12�Ķ�Ԫǿ��������ϣ�c(OH��)��c(H��)
��pH��12�Ķ�Ԫǿ��������ϣ�c(OH��)��c(H��)
D��pH��4Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COOH��CH3COONa�����Һ�У�c(CH3COO��)��c(OH��)��c(CH3COOH)��c(H+)
��ϰ��ϵ�д�
�����Ŀ


 ����Һ���ٴ��ᡢ�����ᡢ�۱����ơ��ܱ��ӡ���̼����
����Һ���ٴ��ᡢ�����ᡢ�۱����ơ��ܱ��ӡ���̼���� ����̼������
����̼������ �������ᡢ���������ƣ�����ҺpH��С����������ȷ����
�������ᡢ���������ƣ�����ҺpH��С����������ȷ���� CH3OH(g)+H2O(g) ��H=-49.0kJ��mol-1
CH3OH(g)+H2O(g) ��H=-49.0kJ��mol-1 ��ʾ����1 minʱH2�����ʵ�����6 mol��
��ʾ����1 minʱH2�����ʵ�����6 mol��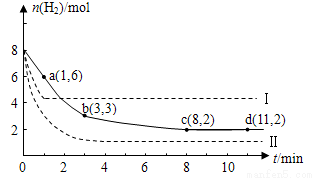
 )�۵�-58�棬�е�126��129�棬������_____________���塣
)�۵�-58�棬�е�126��129�棬������_____________���塣

 HSO3��Һ�У�c��HSO
HSO3��Һ�У�c��HSO ����2c��SO
����2c��SO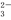 ����c��OH������c��H+����0.1 mol��L��1
����c��OH������c��H+����0.1 mol��L��1