��Ŀ����
���ǵؿ��к�����ߵĽ���Ԫ�أ��䵥�ʼ��Ͻ������������е�Ӧ�������㷺��
���̼�Ȼ�ԭ���Ȼ�����ʵ�����������Ʊ�������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
Al2O3(s)��AlCl3(g)��3C(s)=3AlCl(g)��3CO(g)
��H��a kJ��mol��1
3AlCl(g)=2Al(l)��AlCl3(g)��H��b kJ��mol��1
(1)��ӦAl2O3(s)��3C(s)=2Al(l)��3CO(g)�Ħ�H��________kJ��mol��1(�ú�a��b�Ĵ���ʽ��ʾ)��
(2)Al4C3�Ƿ�Ӧ�����е��м���Al4C3�����ᷴӦ(����֮һ�Ǻ�������ߵ���)�Ļ�ѧ����ʽΪ______________________________________��
��(1)a��b��(2)Al4C3��12HCl=4AlCl3��3CH4��
����

2013��12��15��4ʱ���س���ϵ�л���ġ����úš�˳��ʻ��������棬ʵ�������Ǻ���ҫ����Ĵ��١����������Ҫ����ȼ�ϣ�ͨ�����£�N2H4����ȼ�ϣ�N2O4������������ش��������⣺
��1����֪��N2(g) + 2O2(g) ="=" 2NO2(g) ��H= + 67.7kJ��mol-1
N2H4(g) + O2��g��="=" N2(g) + 2H2O(g) ��H= - 534.0kJ��mol-1
2NO2(g) 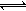 N2O4��g�� ��H=" -" 52.7kJ��mol-1
N2O4��g�� ��H=" -" 52.7kJ��mol-1
д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ҵ���ô�������������İ�����Ӧ�Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3����ҵ�Ͽ��������з�Ӧԭ���Ʊ�������
2N2(g)+6H2O(l)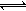 4NH3(g)+3O2(g) ��H= Q kJ��mol-1
4NH3(g)+3O2(g) ��H= Q kJ��mol-1
����֪�÷�Ӧ��ƽ�ⳣ��K���¶ȵĹ�ϵ��ͼ����˷�Ӧ�� Q 0 ���������������=������
������ʼ���뵪����ˮ��15���Ӻ�Ӧ�ﵽƽ�⣬��ʱNH3��Ũ��Ϊ0.3mol/L������������ʾ�ķ�Ӧ����Ϊ ��
�۳����£����������Ӧ�����������ܱ������з���������Ӧ�ﵽƽ��ʱ�� ��ѡ���ţ�.
| A�������������ƽ����Է�����������ʱ����仯 |
| B��v��N2��/v��O2��=2��3 |
| C��������������ܶȲ���ʱ����仯 |
| D��ͨ��ϡ����������߷�Ӧ������ |
��4���������������������õ�ⷨ��H2O2���Դ˴����ϰ�ˮ��װ����ͼ��
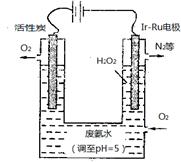
��Ϊ��Ӱ��H2O2�IJ�������Ҫ��ϰ�ˮ�����������������Һ��pHԼΪ5�������÷ϰ�ˮ��Һ��c(NH4+) c(NO3-)���������������=������
��Ir��Ru���Ե缫������O2���ã��õ缫�ĵ缫��ӦΪ ��
�������ϵ�·��ÿת��3mol���ӣ������Դ���NH3��H2O�����ʵ���Ϊ ��
�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ��CO��g����2H2��g��=CH3OH��g������H1
��Ӧ��CO2��g����3H2��g��=CH3OH��g����H2O��g������H2
��������Ӧ���ϡ�ԭ�Ӿ��á�ԭ�����________�������
���±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| �¶� | 250 �� | 300 �� | 350 �� |
| K | 2.041 | 0.270 | 0.012 |
�ɱ��������жϣ���H1______0�����������������������
��ij�¶��£���2 mol CO��6 mol H2����2 L���ܱ������У���ַ�Ӧ���ﵽƽ����c��CO����0.2 mol��L��1����CO��ת����Ϊ________����ʱ���¶�Ϊ________�����ϱ���ѡ��
��2����֪�ڳ��³�ѹ�£�
��2CH3OH��l����3O2��g��=2CO2��g����4H2O��g����H1����1 275.6 kJ��mol��1
��2CO��g����O2��g��=2CO2��g����H2����566.0 kJ��mol��1
��H2O��g��=H2O��l������H3����44.0 kJ��mol��1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��__________________________��
�״���һ����Ҫ�Ļ���ԭ�ϡ��״���ˮ�����������ɻ�������Դ�����й㷺��Ӧ��ǰ������������ʵ�飬�����Ϊ1 L���ܱ������У�����1mol CH3OH��1molH2O��һ�������·�����Ӧ��CH3OH (g)+ H2O (g) CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��
CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��
| ʱ�� ���� | 0 min | 10 min | 30 min | 60 min | 70 min |
| CO2(mol/L) | 0 | 0.2 | 0.6 | 0.8 | 0.8 |
| CH3OH(mol/L) | 1.0 | 0.8 | 0.4 | 0.2 | 0.2 |
����֪��CH3OH (g)+
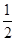 O2 (g)
O2 (g) CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol
CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol H2(g)+
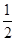 O2 (g)
O2 (g) H2 O(g) ?H2= ��120.9kJ/mol
H2 O(g) ?H2= ��120.9kJ/mol ��״���ˮ������������Ӧ���ʱ��H3=_____ ��
��10~30 min�ڣ�������ƽ����Ӧ����v(H2)��___________mol/(L��min)��
�۸÷�Ӧ��ƽ�ⳣ������ʽΪK=__________________��
�����д�ʩ����ʹƽ��ʱn(CH3OH)��n(CO2)��С����(˫ѡ)___________��
A��������� B�����ݳ���He(g)��ʹ��ϵѹǿ����
C����H2(g)����ϵ�з��� D���ٳ���1molH2O
��2���״��ڴ��������¿���ֱ�������ɼ��ᡣ
���ڳ����£���0.1000 mol/L NaOH��Һ�ζ�20. 00 mL 0.1000 mol/L ������Һ�����У������Һ��pH=7ʱ�������ĵ�V(NaOH)___(�������������) 20. 00 mL��
���������ζ������У��������ỻ�����ᣬ����ͼ�е���Ӧλ�û�����Ӧ�ĵζ����ߡ�(1����ҺԼ0.04mL)
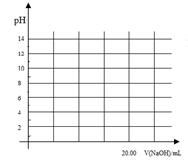
���������ѳ�Ϊ��ǰ��δ����һ��ȫ�����ش���⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��
���̢�������·�Ӧ��ʾ��
��2CO2 2CO��O2����2H2O===2H2��O2����2N2��6H2O
2CO��O2����2H2O===2H2��O2����2N2��6H2O 4NH3��3O2����2CO2��4H2O
4NH3��3O2����2CO2��4H2O 2CH3OH��3O2����2CO��4H2O
2CH3OH��3O2����2CO��4H2O ________��3O2
________��3O2
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ɵڢݸ���Ӧ�Ļ�ѧ����ʽ��____________________��
(3)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(4)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O===O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
�����£�N2��H2O��Ӧ����NH3���Ȼ�ѧ����ʽΪ_________��
�����Ź㷺����;�������ڻ��ʡ����ᡢ�ϳ���ά�ȹ�ҵ������
��1���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɰ�����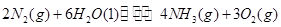
�÷�Ӧ�ڹ̶�������ܱ������н��У��й�˵����ȷ����_____________���������ĸ����
A����Ӧ����ƽ��״̬ʱ��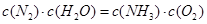 |
B����Ӧ�ﵽƽ���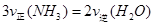 |
| C����ϵ����ѹǿ���䣬˵����Ӧ�Ѵ�ƽ�� |
| D�����������ܶȱ��ֲ��䣬˵����Ӧ�Ѵ�ƽ�� |
 ��
��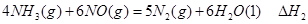 ��
��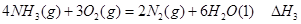 ��
����д������������Ӧ��
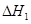 ��
��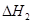 ��
��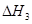 ����֮���ϵ�ı���ʽ��
����֮���ϵ�ı���ʽ��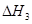 ��_________��
��_________����3����ҵ���������Ҫ��Ӧ�ǣ�
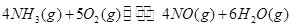
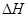 ��
��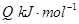
�������¶ȣ���Ӧ��Kֵ��С����Q______���>������<��������0��
������Ӧ��ʼ�����ʵ�����ͬ�����й�ϵͼ�������________������ţ���


�����ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ�Ũ�����±���
| ʱ��/Ũ�� |  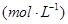 | 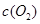 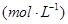 | 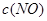 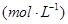 | 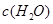 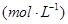 |
| ��ʼ | 4.0 | 5.5 | 0 | 0 |
| ��2min | 3.2 | a | 0.8 | 1.2 |
| ��4min | 2.0 | 3.0 | 2.0 | 3.0 |
| ��6min | 2.0 | 3.0 | 2.0 | 3.0 |
��Ӧ�ڵ�2 min����4 minʱ��O2��ƽ����Ӧ����Ϊ________��
��Ӧ�ڵ�2 minʱ�ı����������ı������������______________________________��
�������£���Ӧ��ƽ�ⳣ��K��________��
 CH3OH��g������H1����90 kJ��mol��1
CH3OH��g������H1����90 kJ��mol��1
 2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
2Fe(s)��3CO(g)����H����492.7 kJ��mol��1 CO(g)��3H2(g)����H����206.2 kJ��mol��1
CO(g)��3H2(g)����H����206.2 kJ��mol��1