��Ŀ����
(7��)��0.5mol/L��NaHSO3��Һ�е���ʯ���Һ��졣�Իش��������⣺
��1������Һ��HSO3���ĵ���̶�______������ڡ�����С�ڡ����ڡ���HSO3����ˮ��̶ȡ�
��2����Һ��Na+��HSO3-��H+��OH-��SO32-�����ӵ�Ũ���ɴ�С��˳��Ϊ ��
��3����Na2SO3��Һ�е����̪����Һ��졣���ڸ���Һ���ٵ��������BaCl2��Һ�����۲쵽�������� ����ԭ���ǣ������ӷ���ʽ����Ҫ����˵���� ��
��1������Һ��HSO3���ĵ���̶�______������ڡ�����С�ڡ����ڡ���HSO3����ˮ��̶ȡ�
��2����Һ��Na+��HSO3-��H+��OH-��SO32-�����ӵ�Ũ���ɴ�С��˳��Ϊ ��
��3����Na2SO3��Һ�е����̪����Һ��졣���ڸ���Һ���ٵ��������BaCl2��Һ�����۲쵽�������� ����ԭ���ǣ������ӷ���ʽ����Ҫ����˵���� ��
��7�֣�ÿ��2�֣���1������(1��)
��2��c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)
��3��������ɫ�������Һ�ɫ��ȥ
��Na2SO3��Һ�У�SO32-ˮ�⣺SO32-+H2O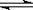 HSO3-+OH-������BaCl2��
HSO3-+OH-������BaCl2��
Ba2++SO32-=BaSO3������ɫ��,����c(SO32-)��С��SO32-ˮ��ƽ�����ƣ�c(OH-)��С����̪��ɫ��
��2��c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)
��3��������ɫ�������Һ�ɫ��ȥ
��Na2SO3��Һ�У�SO32-ˮ�⣺SO32-+H2O
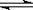 HSO3-+OH-������BaCl2��
HSO3-+OH-������BaCl2��Ba2++SO32-=BaSO3������ɫ��,����c(SO32-)��С��SO32-ˮ��ƽ�����ƣ�c(OH-)��С����̪��ɫ��
��

��ϰ��ϵ�д�
 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�
�����Ŀ

 ���еμ�KOH��Һ����ַ�Ӧ�˳������ã�
���еμ�KOH��Һ����ַ�Ӧ�˳������ã�  H3O++S2��
H3O++S2��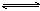 H2S+OH��
H2S+OH��